Raltegravir, sold under the brand name Isentress, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS. It may also...
17 KB (1,458 words) - 17:06, 24 September 2024
for raltegravir (brand name Isentress). Research published at the time supported the conclusion that "[for people living with HIV,] raltegravir plus...
6 KB (622 words) - 17:42, 23 December 2023
Lamivudine/raltegravir, sold under the brand name Dutrebis, is a fixed-dose combination antiretroviral medication used in the treatment of HIV/AIDS. It...
3 KB (136 words) - 19:50, 20 December 2023
of tenofovir disoproxil to be taken once daily, and 400 mg pills of raltegravir to be taken twice daily. People initiating nPEP treatment typically receive...
31 KB (3,370 words) - 15:50, 1 September 2024
Indinavir Lamivudine (3TC) Lopinavir Maraviroc Nelfinavir Nevirapine Raltegravir Ritonavir Saquinavir Tenofovir Tipranavir Zalcitabine (ddC) Zidovudine...
26 KB (1,722 words) - 09:50, 2 November 2024
all known and appear in a variety of pharmaceutical drugs including raltegravir, butalamine, fasiplon, oxolamine, and pleconaril. The 1,2,3-isomer is...
2 KB (152 words) - 20:09, 22 July 2019
Zanamivir J05AH02 Oseltamivir J05AH03 Peramivir J05AH04 Laninamivir J05AJ01 Raltegravir J05AJ02 Elvitegravir J05AJ03 Dolutegravir J05AJ04 Cabotegravir J05AP01...
6 KB (538 words) - 04:59, 18 December 2023
Darunavir Lopinavir/ritonavir (lopinavir + ritonavir) Ritonavir Dolutegravir Raltegravir Abacavir/lamivudine (abacavir + lamivudine) Dolutegravir/lamivudine/tenofovir...
67 KB (4,822 words) - 18:15, 10 November 2024
under clinical trial,[when?] and raltegravir became the first to receive FDA approval in October 2007. Raltegravir has two metal binding groups that...
133 KB (15,784 words) - 05:42, 17 September 2024
2020 revenue. Other major products by the company include Isentress (raltegravir), an antiretroviral medication used to treat HIV/AIDS that had $857 million...
99 KB (9,725 words) - 22:20, 5 November 2024
United States consists of three medications—tenofovir, emtricitabine and raltegravir—as this may reduce the risk further. PEP treatment is recommended after...
211 KB (21,185 words) - 04:17, 28 October 2024
3.54% Johnson & Johnson HIV infection Jun-2006 Mon-20XX 61 Isentress Raltegravir 248,924 0.72% Merck & Co., Inc. HIV Infection Dec-1998 Mon-20XX 62 Janumet...
29 KB (281 words) - 06:29, 12 August 2024
rafoxanide (INN) ralitoline (INN) ralitrexed (INN) raloxifene (INN) raltegravir (USAN) ramatroban (INN) ramciclane (INN) ramelteon (USAN) ramifenazone...
5 KB (286 words) - 04:52, 6 September 2024
Pleconaril Picornavirus Schering-Plough Podophyllotoxin Genital wart Raltegravir HIV Integrase inhibitor Remdesivir COVID-19 viral RNA polymerase inhibitor...
13 KB (513 words) - 14:31, 4 May 2024
Bictegravir (BIC) Cabotegravir (CAB) Dolutegravir (DTG)# Elvitegravir (EVG) Raltegravir (RAL)# BI 224436† MK-2048† Maturation inhibitors Bevirimat† BMS-955176§...
15 KB (1,230 words) - 02:38, 16 July 2024
Bictegravir (BIC) Cabotegravir (CAB) Dolutegravir (DTG)# Elvitegravir (EVG) Raltegravir (RAL)# BI 224436† MK-2048† Maturation inhibitors Bevirimat† BMS-955176§...
28 KB (2,975 words) - 02:18, 11 August 2024
into the host cell genome. Examples of integrase inhibitors include raltegravir, elvitegravir, and dolutegravir. Once a virus genome becomes operational...
55 KB (6,733 words) - 21:23, 24 October 2024
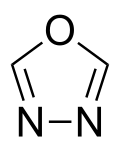
organic chemistry, but many of its derivatives are important. For example, raltegravir is an HIV drug which contains an 1,3,4-oxadiazole ring. Other pharmaceutical...
4 KB (303 words) - 23:09, 2 October 2021
Food and Drug Administration (U.S.) approved the integrase inhibitor Raltegravir (MK-0518, brand name Isentress). The second integrase inhibitor, elvitegravir...
12 KB (1,288 words) - 02:08, 1 June 2024
Laboratory, Chilton, Oxfordshire, England, UK; a national research lab Raltegravir, an antiretroviral medication Zearalenone, an estrogenic metabolite Ral...
2 KB (348 words) - 14:53, 13 June 2023
peptide HIV entry inhibitor Nonbenzodiazepines like zolpidem and zopiclone Raltegravir, an HIV integrase inhibitor SSRIs (selective serotonin reuptake inhibitors)...
44 KB (5,022 words) - 08:47, 12 October 2024
integration, eventually leading to HIV integrase inhibitors such as raltegravir in 2008. Additionally, Montano et al. created a crystal structure of...
7 KB (781 words) - 15:48, 31 March 2024
PMC 2586907. PMID 18558886. Hicks, Charles; Gulick, Roy M. (2009-04-01). "Raltegravir: The First HIV Type 1 Integrase Inhibitor". Clinical Infectious Diseases...
12 KB (1,466 words) - 07:15, 22 August 2024
Flexner, C.; Goh, B. C. (2011). "Simultaneous determination of raltegravir and raltegravir glucuronide in human plasma by liquid chromatography-tandem mass...
13 KB (1,572 words) - 23:39, 18 July 2024
inhibitor, raltegravir, in that "MK-2048 has a dissociation half-life of 32 hours on wild-type integrase—more than four times that of raltegravir", and its...
5 KB (393 words) - 21:08, 27 May 2024
This makes the drug distinct in its mechanism of action compared to raltegravir and elvitegravir, which bind at the catalytic site. In October 2011,...
4 KB (263 words) - 20:41, 1 December 2023
of the first HIV integrase inhibitor to gain FDA approval, Isentress (raltegravir). Her lab also determined these inhibitors' mechanism of action and ways...
14 KB (1,465 words) - 05:55, 16 April 2024
ciclosporin, darunavir/ritonavir, efavirenz, emtricitabine, methadone, raltegravir, rilpivirine, tacrolimus, or tenofovir disoproxil, were evaluated in...
64 KB (5,828 words) - 23:44, 21 October 2024
of drugs used in the treatment of HIV. The first integrase inhibitor, raltegravir, was approved in 2007 and other drugs were in clinical trials in 2011...
32 KB (4,154 words) - 08:34, 29 November 2023
Lessard, Serge Dufresne, and Richard Lalonde. "Switch from enfuvirtide to raltegravir in patients with undetectable viral load: efficacy and safety at 24 weeks...
10 KB (679 words) - 16:53, 26 September 2024