Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), SO2− 3. The sulfite ion is...
19 KB (1,745 words) - 03:46, 27 September 2024
Potassium sulfite is the inorganic compound with the formula K2SO3. It is the salt of potassium cation and sulfite anion. It is a white solid that is highly...
3 KB (164 words) - 14:56, 12 August 2024
Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2SO3. A white, water-soluble solid, it is used commercially as...
7 KB (615 words) - 14:55, 12 August 2024
Lithium sulfite, or lithium sulphite, is an ionic compound with the formula Li2SO3. Fujita, Yushi; Motohashi, Kota; Ding, Jiong; Tsukasaki, Hirofumi;...
2 KB (67 words) - 17:31, 31 October 2024
Barium sulfite is the inorganic compound with the chemical formula BaSO3. It is a white powder that finds few applications. It is an intermediate in the...
3 KB (104 words) - 14:58, 12 August 2024
Sulfite oxidase (EC 1.8.3.1) is an enzyme in the mitochondria of all eukaryotes, with exception of the yeasts.[citation needed] It oxidizes sulfite to...
13 KB (1,683 words) - 17:02, 1 March 2024
Calcium sulfite, or calcium sulphite, is a chemical compound, the calcium salt of sulfite with the formula CaSO3·x(H2O). Two crystalline forms are known...
8 KB (743 words) - 18:02, 11 September 2024
A sulfite sulfate is a chemical compound that contains both sulfite and sulfate anions [SO3]2− [SO4]2−. These compounds were discovered in the 1980s as...
17 KB (1,520 words) - 14:59, 12 August 2024
The sulfite process produces wood pulp that is almost pure cellulose fibers by treating wood chips with solutions of sulfite and bisulfite ions. These...
13 KB (1,535 words) - 00:20, 25 January 2023
Ammonium sulfite is the ammonium salt of sulfurous acid with the chemical formula (NH4)2SO3. Ammonium sulfite can be prepared by the reaction of ammonia...
6 KB (413 words) - 14:59, 12 August 2024
Magnesium sulfite is the magnesium salt of sulfurous acid with the formula MgSO 3. Its most common hydrated form has 6 water molecules making it a hexahydrate...
2 KB (104 words) - 14:57, 12 August 2024
Sodium bisulfite (redirect from Sodium hydrogen sulfite)
Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite...
14 KB (1,411 words) - 23:09, 30 July 2024
Sulfite reductases (EC 1.8.99.1) are enzymes that participate in sulfur metabolism. They catalyze the reduction of sulfite to hydrogen sulfide and water...
6 KB (603 words) - 13:01, 22 April 2024
The topic of sulfite food and beverage additives covers the application of sulfites in food chemistry. "Sulfite" is jargon that encompasses a variety of...
19 KB (1,862 words) - 14:26, 13 March 2023
Dimethyl sulfite is a sulfite ester with the chemical formula (CH3O)2SO. Dimethyl sulfite is used as an additive in some polymers to prevent oxidation...
4 KB (333 words) - 12:34, 1 January 2022
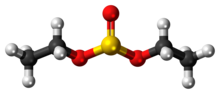
A sulfite ester (also known as an organosulfite) is a functional group with the structure (RO)(R'O)SO. They are in principle the esters of sulfurous acid...
2 KB (238 words) - 09:51, 17 April 2023
Bismuth sulfite agar is a type of agar media used to isolate Salmonella species. It uses glucose as a primary source of carbon. Bismuth and brilliant...
2 KB (131 words) - 03:58, 23 December 2023
Silver sulfite is the chemical compound with the formula Ag2SO3. This unstable silver compound when heated and/or in light it decomposes to silver dithionate...
4 KB (184 words) - 14:58, 12 August 2024
Potassium bisulfite (redirect from Potassium hydrogen sulfite)
Potassium bisulfite (or potassium hydrogen sulfite) is a chemical mixture with the approximately correctly mentioned formula chemical formula KHSO3. Potassium...
4 KB (245 words) - 17:46, 11 September 2024
Dissimilatory sulfite reductase (EC 1.8.99.5) is an enzyme that participates in sulfur metabolism in dissimilatory sulfate reduction. The enzyme is essential...
4 KB (512 words) - 23:53, 6 April 2024
Diethyl sulfite (C4H10O3S) is an ester of sulfurous acid. Among other properties, diethyl sulfite inhibits the growth of mold spores during grain storage...
2 KB (118 words) - 15:36, 19 August 2022
Caramel color (redirect from Sulfite ammonia caramel)
growth unless in a dilute solution. When reacted with sulfites, caramel color may retain traces of sulfite after processing. However, in finished food products...
19 KB (1,791 words) - 05:22, 14 July 2024
of lime results in a slurry of calcium sulfite (CaSO3) that must be disposed of. Fortunately, calcium sulfite can be oxidized to produce by-product gypsum...
26 KB (3,415 words) - 08:40, 17 November 2024
Calcium bisulfite (redirect from Calcium hydrogen sulfite)
Calcium bisulfite (calcium bisulphite or calcium hydrogen sulfite) is an inorganic compound which is the salt of a calcium cation and a bisulfite anion...
8 KB (824 words) - 15:34, 11 September 2024
Isolated sulfite oxidase deficiency is a rare, fatal genetic disease preventing production of sulfite oxidase, needed to metabolize sulfites to sulfates...
99 KB (11,016 words) - 14:18, 8 November 2024
In enzymology, a sulfite dehydrogenase (EC 1.8.2.1) is an enzyme that catalyzes the chemical reaction sulfite + 2 ferricytochrome c + H2O ⇌ {\displaystyle...
2 KB (176 words) - 15:56, 26 August 2023
A chloride sulfite or sulfite chloride is a chemical compound that contains chloride and sulfite anions (SO32− Cl−). The known compounds of this type...
11 KB (576 words) - 16:06, 12 August 2024
Adenylyl-sulfate reductase (redirect from AMP,sulfite:acceptor oxidoreductase (adenosine-5'-phosphosulfate-forming))
(APS) to sulfite through the use of an electron donor cofactor. The products of the reaction are AMP and sulfite, as well as an oxidized...
12 KB (1,533 words) - 16:42, 22 September 2024
Sulfonate (redirect from Strecker sulfite alkylation)
A classic preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of...
5 KB (552 words) - 13:49, 4 September 2024
Sulfur dioxide (section From sulfites)
The reverse reaction occurs upon acidification: H+ + HSO−3 → SO2 + H2O Sulfites results by the action of aqueous base on sulfur dioxide: SO2 + 2 NaOH →...
53 KB (7,366 words) - 22:03, 6 November 2024