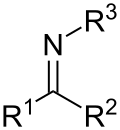
In organosulfur chemistry, sulfinamide is a functional group with the structure R−S(=O)−NR2 (where R = alkyl or aryl). This functionality is composed...
4 KB (430 words) - 15:19, 5 March 2024
Sulfonamide (section Sulfinamides)
organic synthesis. The related sulfinamides (R(S=O)NHR) are amides of sulfinic acids (R(S=O)OH) (see sulfinyl). Chiral sulfinamides such as tert-butanesulfinamide...
8 KB (847 words) - 18:55, 12 March 2024
Tert-Butanesulfinamide (category Sulfinamides)
2-methyl-2-propanesulfinamide or Ellman's sulfinamide) is an organosulfur compound and a member of the class of sulfinamides. Both enantiomeric forms are commercially...
7 KB (440 words) - 14:39, 27 October 2023
Yuanjing; Zhang, Junliang (2020). "Stereoselective Synthesis of Chiral Sulfinamide Monophosphine Ligands (Ming-Phos)(S, Rs)-M". Organic Syntheses. 97: 262–273...
23 KB (2,633 words) - 01:56, 2 September 2024
towards nucleophiles, including thiols. The initial adduct rearranges to a sulfinamide: HNO + RSH → RS(O)NH2 In biological samples, nitroxyl can be detected...
9 KB (898 words) - 02:13, 9 September 2024
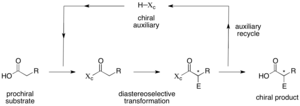
pseudoephedrine amides into synthetically useful functional groups This specific sulfinamide chiral auxiliary was initially developed by Jonathan A. Ellman, and its...
34 KB (3,692 words) - 20:40, 22 July 2024
condensation. Many sulfinamides are commercially available in both (R)- and (S)-forms. The two most commonly used are the Davis p-toluene-sulfinamide and the Ellman...
15 KB (1,583 words) - 03:48, 22 October 2022
, Roy, A., Seibel, W.L., Portlock, D.E. (2003), "Hydroxylamines and sulfinamide as amine components in the Petasis boronic acid–Mannich reaction: synthesis...
29 KB (3,232 words) - 12:16, 21 July 2024
the acid strength and stability diminish in that order. Sulfonamides, sulfinamides and sulfenamides, with formulas R−SO2NR′2, R−S(O)NR′2, and R−SNR′2, respectively...
26 KB (3,035 words) - 15:33, 11 July 2024
useful intermediates for preparation of other sufinyl derivatives such as sulfinamides, sulfinates, sulfoxides, and thiosulfinates. Unlike the sulfur atom in...
6 KB (787 words) - 15:57, 25 July 2023
rubber using sulfur. They are related to the oxidized compounds known as sulfinamides (RS(O)NR2) and sulfonamides (RS(O)2NR2). Sulfenamides are usually prepared...
6 KB (746 words) - 06:37, 11 June 2024
Yuanjing; Zhang, Junliang (2020). "Stereoselective Synthesis of Chiral Sulfinamide Monophosphine Ligands (Ming-Phos)(S, Rs)-M". Organic Syntheses. 97: 262–273...
3 KB (235 words) - 16:08, 5 June 2024
Aspinwall, Craig A.; Miranda, Katrina M. (November 2014). "Glutathione sulfinamide serves as a selective, endogenous biomarker for nitroxyl after exposure...
8 KB (726 words) - 09:34, 17 May 2024
S-hydroxylaminoglutathione, which then rearranges to form glutathione sulfinamide, or in the presence of GSH, forms oxidized glutathione (GSSG) and hydroxyl...
11 KB (1,267 words) - 22:42, 16 May 2024
derivatives. The reagents are commonly called Davis oxaziridines and Davis sulfinamides, respectively. Davis oxidation and Davis' reagent are both named after...
4 KB (350 words) - 00:07, 18 August 2023