Cation–π interaction is a noncovalent molecular interaction between the face of an electron-rich π system (e.g. benzene, ethylene, acetylene) and an adjacent...
35 KB (4,104 words) - 14:03, 23 August 2024
interactions: interaction of anion with π system Cation–π interactions: interaction of a cation with a π system C–H–π interactions: interaction of C-H with π system:...
8 KB (963 words) - 23:44, 5 October 2024
temperature. π-effects can be broken down into numerous categories, including π-stacking, cation-π and anion-π interactions, and polar-π interactions. In general...
27 KB (3,311 words) - 07:44, 12 November 2024
Intermolecular force (redirect from Dipole-dipole interaction)
dipole force Cation–π, σ–π and π–π bonding Van der Waals forces – Keesom force, Debye force, and London dispersion force Cation–cation bonding Salt bridge...
28 KB (3,469 words) - 12:04, 9 October 2024
Dennis A. Dougherty (section Cation-π Interaction)
1998). "From ab initio quantum mechanics to molecular neurobiology: A cation–π binding site in the nicotinic receptor". Proceedings of the National Academy...
12 KB (1,089 words) - 06:14, 5 December 2023
Pi-stacking (section Direct interaction model)
chemistry, pi stacking (also called π–π stacking) refers to the presumptive attractive, noncovalent pi interactions (orbital overlap) between the pi bonds...
32 KB (3,769 words) - 20:04, 3 November 2024
The dihydrogen cation or hydrogen molecular ion is a cation (positive ion) with formula H+ 2. It consists of two hydrogen nuclei (protons), each sharing...
29 KB (3,536 words) - 20:31, 18 October 2024
Stacking (chemistry) (redirect from Aromatic stacking interaction)
generate the [2]catenane product. Noncovalent interaction Dispersion (chemistry) Cation–pi interaction Intercalation (biochemistry) Intercalation (chemistry)...
10 KB (1,041 words) - 18:35, 5 October 2024
Antiaromaticity (redirect from Cyclopentadienyl cation)
change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the diamagnetic ring current present in aromatic compounds...
19 KB (2,236 words) - 04:00, 23 April 2024
of conjugation. Comparison of hydride ion affinities of propyl cation and allyl cation, corrected for inductive effects, results in a considerably lower...
35 KB (4,303 words) - 03:15, 7 October 2024
effectively bind to other surface via hydrophobic interaction and/or cation-π interaction (chitosan as a cation source) in aqueous solution. The free amine...
66 KB (6,749 words) - 17:59, 6 November 2024
2-norbornyl cation (or 2-bicyclo[2.2.1]heptyl cation) describes a carbonium ionic derivative of norbornane. A salt of the 2-norbornyl cation was crystallized...
45 KB (5,145 words) - 13:50, 9 October 2024
Carbenium ion (category Cations)
allyl cation CH2=CH−CH+2 and benzyl cation C6H5−CH+2 are more stable than most other carbenium ions due to donation of electron density from π systems...
25 KB (2,969 words) - 09:14, 9 November 2024
Chronicle. Retrieved 2022-05-26. Lin, Song (2013). Elucidation of the Cation-[pi] Interaction in Small-Molecule Asymmetric Catalysis (Thesis). OCLC 870923011...
13 KB (1,204 words) - 09:09, 18 August 2023
over large distances. Cation-π interactions can also be classified as ionic bonding. This type of interaction occurs when a cation, e.g. acetylcholine,...
6 KB (871 words) - 18:20, 1 August 2024
critical tryptophan ligand binding residue that contributes to a cation-π interaction between the pi electron density of tryptophan and the primary amine...
43 KB (4,628 words) - 10:52, 21 February 2024
π-π, CH/π, S/π, and cation-π interactions. He has developed extensions of symmetry-adapted perturbation theory (SAPT) to analyze these interactions in...
8 KB (763 words) - 20:40, 23 May 2024
Laali, Kenneth K. (2019). "Understanding the interplay between π–π and cation–π interactions in [janusene–Ag] + host–guest systems: a computational approach"...
17 KB (1,857 words) - 21:31, 5 September 2024
relating to the geometry of the crystal; z+ = numeric charge number of cation z− = numeric charge number of anion e = elementary charge, 1.6022×10−19...
5 KB (838 words) - 21:46, 19 July 2024
the contribution of a given interaction up to linear order. For the system, one considers two projectors operators given by Π A = | A ⟩ ⟨ A | , {\displaystyle...
16 KB (2,159 words) - 18:07, 21 November 2024
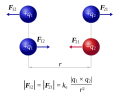
Coulomb's law (redirect from Electrostatic Interaction)
constant of proportionality, 1 4 π ε 0 {\displaystyle {\frac {1}{4\pi \varepsilon _{0}}}} , in Coulomb's law: F 1 = q 1 q 2 4 π ε 0 r ^ 12 | r 12 | 2 {\displaystyle...
41 KB (6,645 words) - 15:38, 27 October 2024
stability and high basicity of the guanidinium cation. Guanidinium does not have a ring structure but has six π-electrons which are delocalized over the molecule...
22 KB (2,764 words) - 20:37, 8 October 2024
The vinyl cation is a carbocation with the positive charge on an alkene carbon. Its empirical formula of the parent ion is C 2H+ 3. Vinyl cation are invoked...
38 KB (4,374 words) - 15:25, 1 September 2024
bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron...
28 KB (3,673 words) - 14:01, 24 October 2024
Electrostatics (redirect from Electrostatic interaction)
\mathbf {r} } (called the field point) of: E ( r ) = 1 4 π ε 0 ∑ i = 1 n q i r ^ i | r i | 2 = 1 4 π ε 0 ∑ i = 1 n q i r i | r i | 3 , {\displaystyle \mathbf...
18 KB (2,508 words) - 23:28, 5 October 2024
triangular cat state: | c a t tri ⟩ ∝ | α ⟩ + | e i 2 π / 3 α ⟩ + | e i 4 π / 3 α ⟩ , {\displaystyle |\mathrm {cat} _{\text{tri}}\rangle \propto |\alpha \rangle...
38 KB (4,629 words) - 00:49, 31 August 2024
together are van der Waals forces, dipole–dipole interactions, quadrupole interactions, π–π interactions, hydrogen bonding, halogen bonding, London dispersion...
33 KB (2,941 words) - 16:03, 25 August 2024
mechanics, the spin–orbit interaction (also called spin–orbit effect or spin–orbit coupling) is a relativistic interaction of a particle's spin with its...
28 KB (4,315 words) - 13:32, 7 November 2024
such as σ → σ*, π → π*, or n → π* meaning excitation of an electron from a σ bonding to a σ antibonding orbital, from a π bonding to a π antibonding orbital...
22 KB (2,830 words) - 18:51, 21 November 2024
inability of aromatic vinylidene substituents to compete with Au for π-interactions since the respective orbitals are perpendicular to each other. Knizia...
25 KB (2,927 words) - 04:10, 4 January 2024