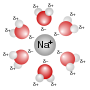
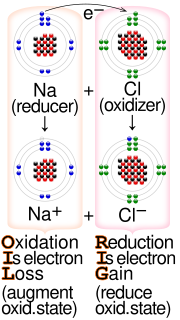
A solvated electron is a free electron in a solution, in which it behaves like an anion. An electron's being solvated in a solution means it is bound...
19 KB (2,068 words) - 17:30, 30 December 2024
be obtained from a linear combination of the four molecular orbitals. Aromatic ring current Electride Solvated electron IUPAC Gold Book delocalization...
4 KB (448 words) - 09:16, 12 February 2024
Oxidizing agent (redirect from Electron acceptors)
Chemical species that donates an electron to another species in a redox reaction Solvated electron – Free electron in a solution, often liquid ammonia...
9 KB (875 words) - 16:12, 12 October 2024
Free electron in physics may refer to: Electron, as a free particle Solvated electron Charge carrier, as carriers of electric charge Valence electron, as...
778 bytes (139 words) - 12:54, 17 January 2021
Hydroxide (redirect from Solvated hydroxide ion)
base by donating a pair of electrons to a Lewis acid. In aqueous solution both hydrogen and hydroxide ions are strongly solvated, with hydrogen bonds between...
41 KB (4,909 words) - 21:46, 5 January 2025
comprising the chain structure of the proteins. Electron equivalent Electrochemical reaction mechanism Solvated electron "Metals". Bitesize. BBC. Archived from...
8 KB (1,005 words) - 04:31, 16 December 2024
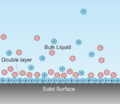
these molecules. Specifically adsorbed, partially solvated ions appear in this layer. The solvated ions of the electrolyte are outside the IHP. Through...
29 KB (3,382 words) - 13:53, 20 October 2024
of standard electrode potential. Nernst equation Pourbaix diagram Solvated electron Standard electrode potential (data page) Standard hydrogen electrode...
8 KB (1,169 words) - 10:34, 11 September 2024
Ion (redirect from Free floating electrons)
is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and...
31 KB (3,178 words) - 08:15, 23 November 2024
("solvates"). For example, crystallization of C60 from benzene solution yields triclinic crystals with the formula C60·4C6H6. Like other solvates, this...
46 KB (4,453 words) - 16:42, 1 January 2025
Photoresist (section Electron-beam exposure)
e− + acid cation + sulfonate anion The e− represents a solvated electron, or a freed electron that may react with other constituents of the solution....
29 KB (3,246 words) - 06:29, 25 November 2024
conduit for electron flow from Cr(II) to Co(III), forming Cr(III) and Co(II). Inner sphere complex Outer sphere electron transfer Solvated electron IUPAC,...
5 KB (636 words) - 12:30, 17 October 2024
stability of the anion-radical and to solvate the cation species formed. The anion-radical can then transfer an electron to the monomer. Initiation can also...
16 KB (1,844 words) - 07:30, 14 December 2024
hydroxydimethylmethyl radical to be converted into acetone and a solvated electron, as the result the G value (yield for a given energy due to radiation...
19 KB (2,422 words) - 02:45, 18 December 2024
Radical anion (redirect from Odd-electron ion)
usually only energetically favorable if the aprotic solvent efficiently solvates the alkali metal ion. Effective solvents are those that bind to the alkali...
9 KB (1,003 words) - 22:45, 14 October 2024
movement of ions, but here we are talking about molten salts rather than solvated ions. In biological membranes, currents are carried by ionic salts. Small...
75 KB (7,984 words) - 07:45, 6 December 2024
Also, some non-aqueous solvents contain Brønsted bases which react with solvated protons. For example, in liquid ammonia, NH2− is the basic ion species...
26 KB (3,041 words) - 06:18, 8 August 2024
with the electron cloud of any available molecule. In aqueous solution, it forms the hydronium ion, H3O+, which in turn is further solvated by water molecules...
68 KB (7,043 words) - 01:50, 8 January 2025
accompanied by an electron charge-transfer between electrolyte and electrode coming from a de-solvated and adsorbed ion. One electron per charge unit is...
25 KB (2,555 words) - 22:37, 4 November 2024
radical absorbance capacity Pourbaix diagram Redox Redox gradient Solvated electron Standard electrode potential Table of standard electrode potentials...
24 KB (3,173 words) - 23:59, 24 February 2024
Lewis acids. It is convention to ignore the fact that a proton is heavily solvated (bound to solvent). With this simplification in mind, acid-base reactions...
22 KB (2,754 words) - 12:04, 2 January 2025
in liquid ammonia, forming ammoniacal solutions of solvated metal cation M+ and solvated electron e−, which react to form hydrogen gas and the alkali...
214 KB (23,524 words) - 08:55, 14 December 2024
"Molecules mimicking atoms: monomers and dimers of alkali metal solvated electron precursors". Physical Chemistry Chemical Physics. 20 (37): 24186–24191...
17 KB (1,847 words) - 00:57, 11 January 2025
Hydrogen (section Electron energy levels)
protons exist freely as a species. To avoid the implication of the naked "solvated proton" in solution, acidic aqueous solutions are sometimes considered...
121 KB (12,377 words) - 14:27, 16 September 2024
electricity through the movement of ions, but not through the movement of electrons. This includes most soluble salts, acids, and bases, dissolved in a polar...
33 KB (3,441 words) - 14:20, 2 January 2025
in the concentration of solvate ions is defined as an acid. A solute that causes an increase in the concentration of the solvate ions and a decrease in...
36 KB (4,969 words) - 11:17, 4 January 2025
Aluminium (section Electron shell)
minute. An aluminium atom has 13 electrons, arranged in an electron configuration of [Ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration...
137 KB (14,842 words) - 03:42, 6 January 2025
potential of the protected metal becomes too negative, reduction of water or solvated protons may evolve hydrogen atoms on the cathode surface, for instance...
15 KB (1,991 words) - 21:19, 18 May 2024
solubility is maximum) yields a triclinic solid solvate C 60·4C 6H 6. Above 30 °C one obtains solvate-free fcc C 60. In 1999, researchers from the University...
61 KB (6,386 words) - 04:20, 9 January 2025