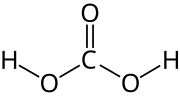
Acid–base homeostasis is the homeostatic regulation of the pH of the body's extracellular fluid (ECF). The proper balance between the acids and bases...
21 KB (2,428 words) - 15:03, 20 November 2024
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton (H+) to a base—in other words,...
13 KB (1,298 words) - 13:45, 10 November 2024
In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several...
36 KB (4,969 words) - 08:10, 10 September 2024
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was first developed by Johannes Nicolaus...
16 KB (1,895 words) - 09:05, 23 November 2024
Acid–base extraction is a subclass of liquid–liquid extractions and involves the separation of chemical species from other acidic or basic compounds....
19 KB (2,204 words) - 14:53, 19 February 2024
fundamental role. In living organisms, acid–base homeostasis and enzyme kinetics are dependent on the pKa values of the many acids and bases present in the cell...
103 KB (11,513 words) - 22:40, 15 October 2024
An acid–base titration is a method of quantitative analysis for determining the concentration of Brønsted-Lowry acid or base (titrate) by neutralizing...
24 KB (2,675 words) - 17:24, 5 November 2024
bicarbonate buffer system, used to maintain acid–base homeostasis. In chemistry, the term "carbonic acid" strictly refers to the chemical compound with...
22 KB (2,178 words) - 00:41, 20 November 2024
Bone health (section Acid–base homeostasis)
to cellular structures or proteins. To maintain homeostasis the body may excrete excess acid or base through the urine, via gas exchange in the lungs...
32 KB (4,046 words) - 04:27, 18 November 2024
organic acids. A few common examples include: Lactic acid Acetic acid Formic acid Citric acid Oxalic acid Uric acid Malic acid Tartaric acid Butyric acid Folic...
10 KB (1,203 words) - 22:29, 14 August 2024
Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming...
22 KB (2,754 words) - 17:47, 24 October 2024
Acid strength is the tendency of an acid, symbolised by the chemical formula HA {\displaystyle {\ce {HA}}} , to dissociate into a proton, H + {\displaystyle...
19 KB (2,596 words) - 17:05, 11 October 2024
a base or acid. For example, with base different aluminate salts will be formed: Al2O3 + 2 NaOH + 3 H2O → 2 NaAl(OH)4 Silicon dioxide is an acidic oxide...
5 KB (580 words) - 02:09, 29 April 2024
mineral acids form hydrogen ions and the conjugate base when dissolved in water. Commonly used mineral acids are sulfuric acid (H2SO4), hydrochloric acid (HCl)...
2 KB (246 words) - 22:29, 25 November 2024
Superacid (redirect from Super Acid)
(according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (H2SO4), which has a Hammett acidity function...
12 KB (1,409 words) - 12:26, 24 November 2024
PH indicator (redirect from Acid-base indicator)
"Ind+" for the conjugate acid of the indicator. The ratio of concentration of conjugate acid/base to concentration of the acidic/basic indicator determines...
19 KB (1,670 words) - 15:01, 3 November 2024
Acid–base imbalance is an abnormality of the human body's normal balance of acids and bases that causes the plasma pH to deviate out of the normal range...
9 KB (778 words) - 14:36, 20 November 2024
Renal physiology (section Acid-base)
maintain acid-base homeostasis, which is the maintenance of pH around a relatively stable value. The lungs contribute to acid-base homeostasis by regulating...
22 KB (1,917 words) - 16:47, 9 October 2024
in the blood are elevated above baseline levels, but the body's acid–base homeostasis is maintained. This contrasts with ketoacidosis, an uncontrolled...
25 KB (2,929 words) - 09:30, 2 November 2024
in much the same way as a weak acid does, with a base dissociation constant (Kb) indicating the strength of the base. For example, when ammonia is put...
9 KB (1,217 words) - 22:26, 23 August 2024
of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally...
26 KB (3,041 words) - 06:18, 8 August 2024
moderate strength (pKa of conjugate acid around 10-13) N,N-Diisopropylethylamine (DIPEA, also called Hünig's Base), pKa = 10.75 1,8-Diazabicycloundec-7-ene...
3 KB (354 words) - 02:44, 22 March 2024
environment. Thus, to Barcroft homeostasis was not only organized by the brain—homeostasis served the brain. Homeostasis is an almost exclusively biological...
82 KB (9,873 words) - 17:58, 31 October 2024
The enzyme maintains acid-base balance and helps transport carbon dioxide. Carbonic anhydrase helps maintain acid–base homeostasis, regulate pH, and fluid...
38 KB (4,361 words) - 17:01, 15 November 2024
Solid acids are acids that are insoluble in the reaction medium. They are often used as heterogeneous catalysts. Many solid acids are zeolites. A variety...
5 KB (462 words) - 12:59, 3 October 2024
PH (redirect from Acid and base)
of acid-base balance known as acid–base homeostasis. Acidosis, defined by blood pH below 7.35, is the most common disorder of acid–base homeostasis and...
52 KB (6,447 words) - 15:58, 24 November 2024
biochemical processes such as oxygen transport by hemoglobin in blood and acid–base homeostasis in the human body. Stability constants, formation constants, binding...
42 KB (6,736 words) - 03:07, 15 September 2024
Superbase (redirect from Super base)
can be designed by utilizing various approaches to stabilize the conjugate acid, up to the theoretical limits of basicity. Organometallic superbases, sometimes...
12 KB (1,181 words) - 12:50, 14 November 2024
Proton affinity (section Acid/base chemistry)
affinity, the stronger the base and the weaker the conjugate acid in the gas phase. The (reportedly) strongest known base is the ortho-diethynylbenzene...
15 KB (1,028 words) - 15:15, 24 April 2024
Kidney (section Acid–base balance)
organ systems that help regulate the body's acid–base balance are the kidneys and lungs. Acid–base homeostasis is the maintenance of pH around a value of...
62 KB (6,985 words) - 10:25, 17 November 2024