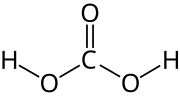
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO− 3), and carbon...
14 KB (1,723 words) - 17:09, 16 November 2024
living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also...
22 KB (2,336 words) - 16:33, 27 August 2024
chemical formula HCO− 3. Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814...
13 KB (1,159 words) - 13:49, 21 December 2024
Carbonic acid (redirect from Hydrogen bicarbonate)
dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis. In chemistry, the term...
22 KB (2,178 words) - 00:41, 20 November 2024
9, ammonium bicarbonate is one of the only options available as the primary buffering agent for most LC-MS buffers. Ammonium bicarbonate is also a key...
12 KB (1,297 words) - 21:42, 16 December 2024
Acid–base homeostasis (redirect from Buffer system)
chemical buffers which minimize pH changes that would otherwise occur in their absence. These buffers include the bicarbonate buffer system, the phosphate...
21 KB (2,428 words) - 15:03, 20 November 2024
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula...
57 KB (5,450 words) - 06:26, 19 December 2024
Homeostasis (redirect from Homeostatic control system)
bicarbonate buffer system can also come into play. Renal compensation can help the bicarbonate buffer system. The sensor for the plasma bicarbonate concentration...
82 KB (9,856 words) - 13:23, 13 December 2024
Metabolic acidosis (section Buffer)
acidity of the blood by four buffering mechanisms.[citation needed] Bicarbonate buffering system Intracellular buffering by absorption of hydrogen atoms...
33 KB (3,850 words) - 13:50, 18 November 2024
Diabetic ketoacidosis (section Sodium bicarbonate)
(metabolic acidosis). The body initially buffers the change with the bicarbonate buffering system, but this system is quickly overwhelmed and other mechanisms...
44 KB (5,086 words) - 23:38, 11 September 2024
carbon dioxide, the latter of which acidifies the blood via the bicarbonate buffer system. The resulting acidity causes the hemoglobin of the blood to lose...
28 KB (3,440 words) - 12:43, 20 November 2024
Henderson–Hasselbalch equation (redirect from Buffer equation)
derived an equation to calculate the hydrogen ion concentration of a bicarbonate buffer solution, which rearranged looks like this: [H+] [HCO3–] = K [CO2]...
17 KB (2,342 words) - 14:58, 11 December 2024
deal of phosphate, amino acids and vitamins. RPMI 1640 uses a bicarbonate buffering system and requires a 5–10% CO2 atmosphere to maintain physiological...
3 KB (445 words) - 09:39, 16 November 2024
sanitation systems. Livestock urine and feces also require proper management if the livestock population density is high. Most animals have excretory systems for...
45 KB (5,473 words) - 16:59, 3 December 2024
calcium level. Sodium bicarbonate is in the alkalinizing family of medications. It works by increasing blood bicarbonate, which buffers excess hydrogen ion...
14 KB (1,012 words) - 01:54, 3 November 2024
Anion gap (redirect from Buffer base)
with disease or intoxication, cause loss of HCO− 3 due to bicarbonate's activity as a buffer (without a concurrent increase in Cl−). Thus, finding a high...
21 KB (2,540 words) - 16:05, 16 November 2024
anaerobic exercise is among others due to factors including the bicarbonate buffer system. The body tries to compensate for the accumulation of lactate...
18 KB (2,287 words) - 16:34, 28 August 2024
the bicarbonate buffer system (HCO3-/CO2), which allows maintaining a constant pH level of the blood and extracellular fluid. This buffer system is described...
82 KB (12,723 words) - 00:50, 18 June 2024
status (above or below pH 7.4). It is slower than the initial bicarbonate buffer system in the blood, but faster than renal compensation. Respiratory...
7 KB (798 words) - 19:02, 18 August 2024
ammonia, while proteins and phosphate act as intracellular buffers. The bicarbonate buffering system is especially key, as carbon dioxide (CO2) can be shifted...
9 KB (778 words) - 14:36, 20 November 2024
of a carbonate or bicarbonate and a weak acid. The base and acid are prevented from reacting prematurely by the inclusion of a buffer such as cornstarch...
48 KB (5,339 words) - 22:08, 7 December 2024
base of acetic acid is acetate. Carbonic acid, which occurs in bicarbonate buffer systems in nature, is not generally classed as one of the carboxylic acids...
26 KB (2,619 words) - 12:07, 27 December 2024
human blood acts as a buffer to maintain pH. The most important buffer in our bloodstream is the carbonic acid-bicarbonate buffer, which prevents drastic...
13 KB (1,298 words) - 13:45, 10 November 2024
physiology: Note that in this equation, the HB/B- buffer system represents all non-bicarbonate buffers present in the blood, such as hemoglobin in its various...
15 KB (1,902 words) - 03:56, 29 January 2023
lead to unwanted side reactions and degradation. A simple sodium bicarbonate buffer system is commonly employed to alleviate this issue, which is especially...
28 KB (3,396 words) - 17:19, 26 September 2024
present, and a chronic phase is entered with partial buffering of the acidosis through renal bicarbonate retention.[citation needed] However, in cases where...
11 KB (1,310 words) - 14:55, 20 November 2024
feedback systems to increase production when needed, such as after a meal. Other cells in the stomach produce bicarbonate, a base, to buffer the fluid...
15 KB (1,772 words) - 19:41, 3 December 2024
waters contain added or dissolved minerals such as potassium bicarbonate, sodium bicarbonate, sodium citrate, or potassium sulfate. These occur naturally...
36 KB (3,960 words) - 06:27, 24 December 2024
Sodium acetate (section Buffer solution)
solution known as vinegar, with sodium carbonate ("washing soda"), sodium bicarbonate ("baking soda"), or sodium hydroxide ("lye", or "caustic soda"). Any...
16 KB (1,143 words) - 13:20, 1 January 2025
medication. It works by decreasing the formation of hydrogen ions and bicarbonate from carbon dioxide and water. Acetazolamide came into medical use in...
27 KB (2,597 words) - 18:02, 9 December 2024