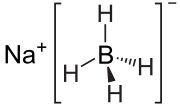
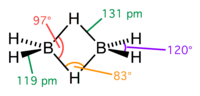
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4 (sometimes written...
27 KB (2,615 words) - 13:44, 24 January 2024
Borohydride refers to the anion [BH4]−, which is also called tetrahydridoborate, and its salts. Borohydride or hydroborate is also the term used for compounds...
8 KB (816 words) - 15:09, 10 September 2024
Lithium borohydride (LiBH4) is a borohydride and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium...
10 KB (827 words) - 02:07, 31 October 2023
Unlike most other metal–borohydrides, which are ionic structures, aluminium borohydride is a covalent compound. Aluminium borohydride is formed by the reaction...
5 KB (301 words) - 18:07, 15 October 2024
Beryllium borohydride is an inorganic compound with the chemical formula Be[BH4]2. Beryllium borohydride is formed by the reaction of beryllium hydride...
3 KB (202 words) - 21:46, 4 April 2024
nonreactive with certain molten salts such as lithium fluoride, lithium borohydride, and sodium hydride. With a molar mass of 7.95 g/mol, it is the lightest...
19 KB (1,932 words) - 20:03, 23 July 2024
Direct borohydride fuel cells (DBFCs) are a subcategory of alkaline fuel cells which are directly fed by sodium borohydride or potassium borohydride as a...
5 KB (660 words) - 14:11, 5 August 2024
Sodium triacetoxyborohydride (category Borohydrides)
other borohydrides, it is used as a reducing agent in organic synthesis. This colourless salt is prepared by protonolysis of sodium borohydride with acetic...
5 KB (412 words) - 18:02, 14 October 2024
Uranium borohydride is the inorganic compound with the empirical formula U(BH4)4. Two polymeric forms are known, as well as a monomeric derivative that...
5 KB (476 words) - 01:09, 3 March 2024
informed that there was no longer a need for uranium borohydride, but it appeared that sodium borohydride could be useful in generating hydrogen. They began...
15 KB (1,386 words) - 05:55, 9 June 2024
Alternatively, a small amount of diborane product can be added to form lithium borohydride, which will react with the BF3 to produce more diborane, making the reaction...
27 KB (2,600 words) - 22:12, 17 October 2024
in vacuum. Although not performed industrially, hydrolysis of sodium borohydride Na[BH4] with a suitable catalyst gives sodium metaborate and hydrogen...
13 KB (1,436 words) - 01:33, 15 August 2023
Reitz AB (April 2004). "Reductive aminations of carbonyl compounds with borohydride and borane reducing agents". Organic Reactions. 59. Hoboken, New Jersey...
165 KB (15,851 words) - 21:46, 14 October 2024
Reductive amination (section Sodium Borohydride)
be isolated and reduced with a suitable reducing agent (e.g., sodium borohydride) to produce the final amine product. Intramolecular reductive amination...
21 KB (2,134 words) - 19:54, 6 October 2024
reagents. Prominent among these reagents are the alkali metal salts of borohydrides and aluminium hydrides. In terms of reaction mechanism, metal hydrides...
24 KB (2,715 words) - 04:43, 3 September 2024
wool, either as gas or from solutions of sodium dithionite, and sodium borohydride. Bleaches generally react with many other organic substances besides...
36 KB (3,891 words) - 01:50, 27 September 2024
catalysts are typically prepared by reacting a salt of nickel with sodium borohydride. The composition and properties vary depending on the specific preparation...
13 KB (1,533 words) - 19:12, 13 September 2023
Stoichiometric reductions are also popular, as can be effected with sodium borohydride. The formyl group readily oxidizes to the corresponding carboxyl group...
30 KB (3,074 words) - 15:22, 14 October 2024
Sodium cyanoborohydride (category Borohydrides)
For example, Na[BH3(CN)] is less reducing than its counterpart sodium borohydride, containing [BH4]−. Sodium cyanoborohydride is a mild reducing agent...
9 KB (792 words) - 20:40, 6 October 2024
reacting with sulfur, phosphorus, phosphorus halides, and potassium borohydride. It dissolves exothermically in water to form dark-green solutions that...
117 KB (13,065 words) - 13:36, 1 October 2024
Nozaki-Hiyama reaction. Aldehydes or ketones are reduced with sodium borohydride or lithium aluminium hydride (after an acidic workup). Another reduction...
35 KB (3,828 words) - 01:07, 19 October 2024
process (see direct borohydride fuel cell) creates hydrogen as needed, but has other issues, such as the high price of the sodium borohydride that is the raw...
101 KB (13,143 words) - 17:43, 2 October 2024
boryllides are extraordinarily rare. Strong bases do not deprotonate a borohydride R2BH to the boryl anion R2B−, instead forming the octet-complete adduct...
127 KB (13,548 words) - 16:02, 23 September 2024
by controlled potential reduction, or chemical reduction using sodium borohydride in alkaline solution, zinc in acetic acid, or by the action of thiols...
30 KB (2,659 words) - 07:33, 30 September 2024
reducing agent. It is more powerful than the related reagent sodium borohydride owing to the weaker Al-H bond compared to the B-H bond. Often as a solution...
35 KB (3,052 words) - 14:36, 12 July 2024
require sodium borohydride as a stabilizer, which could result in undesired side reactions. In contrast, BH3·THF requires sodium borohydride to inhibit reduction...
7 KB (591 words) - 22:09, 24 April 2024
benzaldehyde and benzyl alcohol is possible using DIBAL-H, LiAlH4 or sodium borohydride. Decarboxylation to benzene may be effected by heating in quinoline in...
28 KB (2,451 words) - 05:26, 11 October 2024
with a green flame. It is an intermediate in the preparation of sodium borohydride and is a popular reagent in organic chemistry. It is a weak Lewis acid...
4 KB (355 words) - 11:33, 12 June 2024
mild hydride-reducing agents, such as sodium borohydride, potassium borohydride, and lithium borohydride to dihydroartemisinin (a lactol) in over 90%...
10 KB (998 words) - 09:49, 1 July 2024
camphorquinone . Camphor can also be reduced to isoborneol using sodium borohydride. In biosynthesis, camphor is produced from geranyl pyrophosphate, via...
32 KB (3,043 words) - 00:03, 6 October 2024