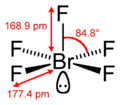
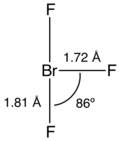
Bromine pentafluoride, BrF5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. BrF5 finds use in oxygen isotope...
6 KB (469 words) - 22:15, 1 January 2025
disproportionating quickly and irreversibly into bromine, bromine trifluoride, and bromine pentafluoride. It thus cannot be obtained pure. It may be synthesised...
68 KB (7,807 words) - 17:58, 28 December 2024
water and most metals and nonmetals. Bromine pentafluoride (BrF5) is a colourless fuming liquid, made by reacting bromine trifluoride with fluorine at 200 °C...
20 KB (2,322 words) - 16:38, 1 November 2024
This page provides supplementary chemical data on bromine pentafluoride. The handling of this chemical may incur notable safety precautions. It is highly...
4 KB (66 words) - 11:53, 12 April 2023
disproportionating quickly and irreversibly into bromine, bromine trifluoride, and bromine pentafluoride. It thus cannot be obtained pure. It may be synthesised...
20 KB (2,374 words) - 09:41, 25 October 2023
Pentafluoride may refer to: Antimony pentafluoride, SbF5 Arsenic pentafluoride, AsF5 Bismuth pentafluoride, BiF5 Bromine pentafluoride, BrF5 Chlorine pentafluoride...
1 KB (121 words) - 09:45, 22 July 2024
Bromine fluoride may refer to several compounds with the elements bromine and fluorine: Bromine monofluoride, BrF Bromine trifluoride, BrF3 Bromine pentafluoride...
533 bytes (56 words) - 20:27, 19 February 2017
produced through the reaction of bromine trifluoride (or bromine pentafluoride) and bromine. Due to its lability, the compound can be detected but not...
3 KB (269 words) - 20:54, 25 September 2023
fluorinating agent, behind only chlorine trifluoride, chlorine pentafluoride, and bromine pentafluoride among the interhalogens: it reacts with almost all the...
104 KB (11,655 words) - 21:58, 31 December 2024
Protactinium(V) fluoride (redirect from Protactinium pentafluoride)
can be obtained by reacting protactinium(V) oxide with bromine trifluoride or bromine pentafluoride at 600 °C: 3 Pa 2 O 5 + 10 BrF 3 ( 6 BrF 5 ) ⟶ 6 PaF...
4 KB (377 words) - 20:06, 12 May 2024
tribromide – BBr3 Bromic acid – HBrO3 Bromine monoxide – Br2O Bromine pentafluoride – BrF5 Bromine trifluoride – BrF3 Bromine monofluoride – BrF Calcium bromide...
119 KB (8,721 words) - 20:49, 6 December 2024
Bromine dioxide is the chemical compound composed of bromine and oxygen with the formula BrO2. It forms unstable yellow to yellow-orange crystals. It was...
4 KB (245 words) - 13:24, 20 December 2023
molecules with this structure include chlorine pentafluoride, bromine pentafluoride, and iodine pentafluoride. The base of a square pyramid can be attached...
24 KB (2,528 words) - 18:47, 18 November 2024
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure...
8 KB (538 words) - 18:29, 21 December 2023
laboratory process is the reaction of lithium nitrate LiNO3 and bromine pentafluoride BrF5, in the ratio exceeding 3:1. The reaction first forms nitryl...
23 KB (2,324 words) - 10:06, 16 November 2024
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes...
7 KB (525 words) - 13:29, 27 September 2024
6, or both. Trace-scale heating of radon with xenon, fluorine, bromine pentafluoride, and either sodium fluoride or nickel fluoride was claimed to produce...
132 KB (14,639 words) - 03:10, 3 January 2025
the help of stronger fluorinating reagents like bromine trifluoride (BrF3) or bromine pentafluoride (BrF5). These reactions can be used to separate plutonium...
13 KB (1,316 words) - 16:58, 10 June 2024
fluorinating agent, behind only chlorine trifluoride, chlorine pentafluoride, and bromine pentafluoride among the interhalogens: it reacts with almost all the...
30 KB (3,558 words) - 02:08, 12 September 2024
Bromyl fluoride (redirect from Bromine fluoride dioxide)
of BrF5 with IO2F, IOF3, and I2O5 produce BrO2F. Hydrolysis of bromine pentafluoride at low temperatures: BrF5 + 2H2O → BrO2F + 4HF The compound forms...
3 KB (235 words) - 07:23, 3 January 2025
Dibromine pentoxide (redirect from Bromine pentoxide)
Dibromine pentoxide is the chemical compound composed of bromine and oxygen with the formula Br2O5. It is a colorless solid that is stable below −20 °C...
3 KB (125 words) - 02:40, 11 December 2024
hexafluoronickelate is a strong oxidant. It can turn chlorine pentafluoride and bromine pentafluoride into ClF+ 6 and BrF+ 6, respectively: K 2 N i F 6 + 5...
4 KB (410 words) - 12:04, 19 December 2024
12310–58–6 BrCl bromine monochloride 13863–41–7 BrF bromine monofluoride 13863–59–7 BrF3 bromine trifluoride 7787–71–5 BrF5 bromine pentafluoride 7789–30–2...
139 KB (120 words) - 17:07, 15 July 2024
similarly from in-situ generated bromine monofluoride. Nyman, F., Roberts, H. L., Seaton, T. "Sulfur Chloride Pentafluoride" Inorganic Syntheses, 1966, Volume...
4 KB (306 words) - 14:45, 6 February 2024
trifluoride 7637-07-2 250 Bromine 7726-95-6 1500 Bromine chloride 13863-41-7 1500 Bromine pentafluoride 7789-30-2 2500 Bromine trifluoride 7787-71-5 15000...
7 KB (69 words) - 13:43, 24 May 2024
can be prepared by reacting protactinium oxide with either bromine pentafluoride or bromine trifluoride at about 600 °C, and protactinium(IV) fluoride...
50 KB (5,847 words) - 13:00, 5 December 2024
Disulfur decafluoride (redirect from Sulfur pentafluoride)
reacts to form sulfur chloride pentafluoride (SF 5Cl): S 2F 10 + Cl 2 → 2 SF 5Cl The analogous reaction with bromine is reversible and yields SF 5Br...
8 KB (654 words) - 01:34, 10 May 2024
The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between...
117 KB (13,073 words) - 15:40, 1 January 2025
oxidizing power, it is comparable to krypton difluoride. It can oxidize bromine pentafluoride to hexafluorobrome(VII) cation, potassium hexafluoroplatinate(V)...
3 KB (222 words) - 00:26, 23 June 2024
required. Their first joint research paper described the use of bromine pentafluoride to extract oxygen from rocks and minerals. They developed several...
13 KB (1,272 words) - 07:45, 13 September 2024