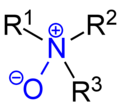
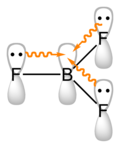
chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which...
10 KB (1,313 words) - 13:10, 17 August 2024
A coordinate covalent bond is a covalent bond in which the two shared bonding electrons are from the same one of the atoms involved in the bond. For...
40 KB (4,872 words) - 13:33, 22 September 2024
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared...
28 KB (3,673 words) - 05:58, 14 October 2024
structure in linguistics Coordinate covalent bond in chemistry Coordinate descent, an algorithm Coordination (disambiguation) Coordinator (disambiguation) This...
515 bytes (86 words) - 08:15, 21 February 2019
triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple...
5 KB (516 words) - 14:46, 15 March 2024
The covalent bond classification (CBC) method, also referred to as LXZ notation, is a way of describing covalent compounds such as organometallic complexes...
6 KB (734 words) - 09:09, 9 January 2024
A silicon–oxygen bond (Si−O bond) is a chemical bond between silicon and oxygen atoms that can be found in many inorganic and organic compounds. In a...
11 KB (1,124 words) - 12:41, 22 September 2024
chemistry, a hydrogen bond (or H-bond) is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a more electronegative...
46 KB (5,459 words) - 23:38, 3 October 2024
structural isomer. convection cooling curve coordinate chemistry coordinate covalent bond See dipolar bond. coordination complex A chemical compound consisting...
171 KB (18,187 words) - 16:16, 23 May 2024
pairs form a crystal structure with metallic bonding between them. Another example of a metal–metal covalent bond is the mercurous ion (Hg2+ 2). As chemistry...
24 KB (3,401 words) - 20:25, 18 January 2024
accuracy of the data given in this table. Atomic radius Covalent radius (Single-, double- and triple-bond radii, up to the superheavy elements.) Ionic radius...
31 KB (863 words) - 21:23, 1 September 2024
be viewed as a coordinate covalent bond, wherein two electrons are donated by the nitrogen atom which acts as the Lewis base to a bond to the Lewis acidic...
127 KB (13,548 words) - 16:02, 23 September 2024
has the chemical formula R3N+−O−. It contains a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substituent-groups attached...
12 KB (1,327 words) - 17:22, 31 July 2024
former, it has been suggested that the carbon atom attaches via a coordinate covalent bond to an oxygen atom from the substrate through its free bonds. In...
5 KB (470 words) - 05:06, 25 September 2024
be viewed as a coordinate covalent bond, wherein two electrons are donated by the nitrogen atom which acts as the Lewis base to a bond to the Lewis acidic...
12 KB (1,320 words) - 09:51, 1 May 2024
Orbital hybridisation (redirect from Sp² bond)
p-type orbitals to form two covalent bonds with two hydrogen atoms in a methylene (CH2) molecule, with a hypothetical bond angle of 90° corresponding to...
33 KB (3,169 words) - 13:02, 22 September 2024
functional group that donates one or more of its electrons through a coordinate covalent bond to one or more central atoms or ions Ligand (biochemistry), a substance...
361 bytes (82 words) - 20:04, 9 January 2019
metal ion affinity chromatography (IMAC) is based on the specific coordinate covalent bond of amino acids, particularly histidine, to metals. This technique...
29 KB (3,430 words) - 03:30, 24 June 2024
reversibly bind oxygen by a coordinate covalent bond, completing the octahedral group of six ligands. This reversible bonding with oxygen is why hemoglobin...
98 KB (11,567 words) - 12:15, 17 September 2024
Coordination complex (redirect from Coordinated complex)
for the bond between ligand and central atom. L ligands provide two electrons from a lone electron pair, resulting in a coordinate covalent bond. X ligands...
57 KB (5,553 words) - 04:29, 2 October 2024
Carboxypeptidase cleaves peptide linkages during digestion of proteins. A coordinate covalent bond is formed between the terminal peptide and a C=O group attached...
143 KB (16,228 words) - 07:01, 9 October 2024
Chemical compound (section Bonding and forces)
together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case...
23 KB (2,643 words) - 14:21, 29 September 2024
electrons come from one of the atoms, it was called a dative covalent bond or coordinate bond. The distinction is not very clear-cut. For example, in the...
22 KB (2,754 words) - 23:27, 18 October 2024
Hypervalent molecule (redirect from Hypervalent bonding)
expected van der Waals value in A (a weak bond) almost to the expected covalent single bond value in C (a strong bond). Corriu and coworkers performed early...
39 KB (4,486 words) - 20:28, 22 July 2024
covalent bond, ionic bonding, coordinate covalent bond, Bond dipole moment, acid-base, redox, DNA Chemistry II Van der Waals force, hydrogen bond, P...
49 KB (4,583 words) - 01:55, 14 September 2024
an acid is a substance that accepts an electron pair to form a coordinate covalent bond. An acid dissociation constant is a particular example of an equilibrium...
103 KB (11,513 words) - 22:40, 15 October 2024
the above example) are now classed as ionic, and each Co-N bond is a coordinate covalent bond between the Lewis acid Co3+ and the Lewis base NH3. Lehrbuch...
9 KB (930 words) - 20:40, 27 June 2024
compound to which electrons are donated during the formation of a coordinate covalent bond Acceptor (semiconductors) Acceptor (finite-state machine), in sequential...
718 bytes (141 words) - 00:34, 14 April 2022
confused with dynamic combinatorial chemistry, DCvC concerns only covalent bonding interactions. As such, it only encompasses a subset of supramolecular...
18 KB (1,997 words) - 12:31, 11 October 2024
Enzyme catalysis (redirect from Covalent catalysis)
the energetics of the covalent bond to the serine molecule in chymotrypsin should be compared to the well-understood covalent bond to the nucleophile in...
43 KB (5,001 words) - 18:45, 7 August 2024