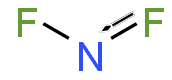
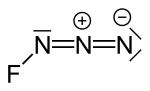Dinitrogen difluoride is a chemical compound with the formula N2F2. It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition...
7 KB (574 words) - 04:35, 14 February 2024
Palladium difluoride Platinum difluoride Silver difluoride Vanadium difluoride Zinc difluoride Lead difluoride Tin(II) fluoride Dinitrogen difluoride Oxygen...
6 KB (436 words) - 19:27, 23 September 2024
Nitrogen (redirect from Dinitrogen)
Fluorine azide (FN3) is very explosive and thermally unstable. Dinitrogen difluoride (N2F2) exists as thermally interconvertible cis and trans isomers...
105 KB (12,228 words) - 16:47, 4 October 2024
fluorine atom. It is unstable with respect to its formal dimer, dinitrogen difluoride, as well as to its elements, nitrogen and fluorine. Nitrogen monofluoride...
4 KB (364 words) - 22:49, 21 July 2024
(Heptafluoropropyl)carbonimidic difluoride 378-00-7 Pentafluoroethyl carbonimidic difluoride 428-71-7 (Trifluoromethyl)carbonimidic difluoride 371-71-1 CF3N=CF2...
96 KB (4,549 words) - 01:43, 14 October 2024
Nitrogen compounds (section Dinitrogen complexes)
Fluorine azide (FN3) is very explosive and thermally unstable. Dinitrogen difluoride (N2F2) exists as thermally interconvertible cis and trans isomers...
34 KB (4,298 words) - 07:31, 17 May 2024
monofluoride, NF Nitrogen difluoride radical, ·NF2 Nitrogen trifluoride, NF3 Nitrogen pentafluoride, NF5 Dinitrogen difluoride, N2F2 Tetrafluorohydrazine...
656 bytes (73 words) - 18:30, 7 June 2023
and alkanes. The dicationic form, H−N+≡N+−H (diazynediium, diprotonated dinitrogen), is calculated to have the strongest known chemical bond. This ion can...
8 KB (606 words) - 05:41, 29 August 2024
inversion center, no vertical plane trans-1,2-dichloroethylene trans-dinitrogen difluoride trans-azobenzene C2v E C2 σv(xz) σv'(yz) angular (H2O) or see-saw...
47 KB (4,053 words) - 15:14, 9 October 2024
Related binary fluoro-azanes tetrafluorohydrazine Related compounds dinitrogen difluoride Except where otherwise noted, data are given for materials in their...
22 KB (1,964 words) - 06:53, 26 September 2024
7727-37-9 N2F2 dinitrogen difluoride 13812–43–6 N2F4 dinitrogen tetrafluoride 10036–47–2 N2O dinitrogen monoxide 10024–97–2 N2O3 dinitrogen trioxide 10544–73–7...
139 KB (120 words) - 17:07, 15 July 2024
Diphosphorus tetrafluoride (redirect from Phosphorus difluoride)
stronger than the corresponding N−N bond in dinitrogen tetrafluoride which easily breaks into nitrogen difluoride. The infrared spectrum has absorption at...
5 KB (482 words) - 14:27, 13 August 2024
azide decomposes without explosion at normal temperatures to make dinitrogen difluoride: 2 FN3 → N2F2 + 2 N2. At higher temperatures such as 1000 °C fluorine...
8 KB (728 words) - 22:43, 21 July 2024
as well as the nitrogen difluoride radical. Another way to make difluoroamino sulfur pentafluoride is by heating dinitrogen tetrafluoride and sulfur...
10 KB (931 words) - 15:28, 2 June 2022
Argon compounds (redirect from Argon difluoride)
argon water clathrate is described in the Aqueous argon section. Argon difluoride, ArF2, is predicted to be stable at pressures over 57 GPa. It should be...
132 KB (15,778 words) - 19:46, 12 May 2024
Tetrafluorohydrazine (redirect from Dinitrogen tetrafluoride)
Tetrafluorohydrazine is in equilibrium with its radical monomer nitrogen difluoride. N2F4 ⇌ 2 •NF2 At room temperature N2F4 is mostly associated with only...
7 KB (634 words) - 23:09, 18 October 2024
Shreeve's work includes novel synthesis of chlorodifluoroamine and dinitrogen difluoride, precursors to rocket propellant oxidizers that were difficult to...
8 KB (908 words) - 01:49, 27 May 2024
Nitrogen difluoride, also known as difluoroamino, is a reactive radical molecule with formula NF2. This small molecule is in equilibrium with its dimer...
9 KB (850 words) - 20:48, 8 February 2024
two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons. The experimental B–F bond length is 1...
14 KB (1,433 words) - 10:51, 23 March 2024
pentachloride – NbCl5 Dinitrogen pentoxide (nitronium nitrate) – N2O5 Dinitrogen tetrafluoride – N2F4 Dinitrogen tetroxide – N2O4 Dinitrogen trioxide – N2O3...
119 KB (8,735 words) - 14:26, 16 September 2024
difluoride, tetrafluoride, hexafluoride, and multiple oxyfluorides have been isolated since then. Among other noble gases, krypton forms a difluoride...
157 KB (15,346 words) - 01:04, 8 September 2024
dithallium difluoride 31970-97-5 F2W tungsten difluoride 33963-15-4 F2Xe xenon difluoride 13709-36-9 F2Y yttrium difluoride 13981-89-0 F2Zn zinc difluoride 7783-49-5...
183 KB (107 words) - 22:11, 6 September 2024
alternate low yield method to make FXeONO2 is to dissolve xenon difluoride in liquid dinitrogen tetroxide at 0 °C. XeF2 + NO+ + NO3− → FXeONO2 + NOF This method...
5 KB (471 words) - 20:37, 4 January 2024