In electrochemistry, an electrochemical reaction mechanism is the step-by-step sequence of elementary steps, involving at least one outer-sphere electron...
9 KB (1,244 words) - 17:31, 1 May 2023
chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs. A chemical mechanism is a theoretical...
13 KB (1,686 words) - 06:29, 9 August 2024
Electron transfer (redirect from Electron-transfer reaction)
describes the mechanism by which electrons are transferred in redox reactions. Electrochemical processes are ET reactions. ET reactions are relevant to...
8 KB (923 words) - 18:07, 31 October 2024
possible to determine even more specific information (see electrochemical reaction mechanism). The current maxima for oxidation and reduction itself depend...
28 KB (3,490 words) - 08:03, 25 October 2024
be found in electrochemical organic synthesis or electrosynthesis. Examples of organic reactions that can take place in an electrochemical cell are the...
6 KB (672 words) - 19:09, 3 March 2024
Electrochemical kinetics is the field of electrochemistry that studies the rate of electrochemical processes. This includes the study of how process conditions...
3 KB (396 words) - 15:30, 20 October 2024
Voltammetry (section Electrochemical cells)
(amperes). Electrochemical cells are used in voltammetric experiments to drive the redox reaction of the analyte. Like other electrochemical cells, two...
29 KB (3,156 words) - 11:44, 21 November 2024
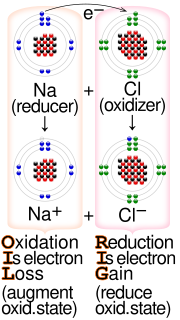
Redox (redirect from Redox reaction)
each called a half-reaction because two half-reactions always occur together to form a whole reaction. In electrochemical reactions the oxidation and reduction...
37 KB (3,716 words) - 07:57, 9 November 2024
driving the electrochemical reaction. In principle, ammonia has an extremely small electrochemical window, but thermodynamically-favored reactions less than...
9 KB (1,052 words) - 14:52, 22 November 2024
An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two...
24 KB (2,770 words) - 23:32, 3 September 2024
Dielectric spectroscopy (redirect from Electrochemical impedance spectroscopy)
Often, EIS reveals information about the reaction mechanism of an electrochemical process: different reaction steps will dominate at certain frequencies...
25 KB (3,097 words) - 14:24, 13 August 2024
Kolbe electrolysis (redirect from Kolbe reaction)
R1−R1 + R1−R2 + R2−R2 + 6 CO2 + 6 e− The reaction mechanism involves a two-stage radical process: electrochemical decarboxylation gives a radical intermediate...
8 KB (649 words) - 16:29, 26 September 2024
Galvanic cell (redirect from Electrical potential of the reaction)
respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. An example of a galvanic...
22 KB (2,995 words) - 05:29, 19 October 2024
Chemical oscillator (redirect from Oscillating reaction)
University Press, USA, 1998, p. 3. Espenson, J.H. Chemical Kinetics and Reaction Mechanisms (2nd ed., McGraw-Hill 2002) p.190 ISBN 0-07-288362-6 "IDEA - Internet...
7 KB (838 words) - 18:27, 4 November 2024
information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically...
66 KB (8,043 words) - 01:08, 14 October 2024
Chemiosmosis (redirect from Chemiosmotic mechanism)
of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate...
25 KB (3,154 words) - 13:29, 22 July 2024
electrode. These working electrodes are used in electrochemical studies when investigating reaction mechanisms related to redox chemistry, among other chemical...
4 KB (506 words) - 00:07, 3 August 2023
The Virtual breakdown mechanism is a concept in the field of electrochemistry. In electrochemical reactions, when the cathode and the anode are close...
11 KB (1,398 words) - 08:02, 11 September 2023
gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated...
50 KB (6,488 words) - 19:29, 22 October 2024
Electrolysis of water (redirect from Electrochemical water splitting)
energy than the minimum. Electrocatalyst Electrochemistry Electrochemical cell Electrochemical engineering Electrolysis Gas cracker Hydrogen production...
77 KB (8,277 words) - 04:31, 24 November 2024
Fenton's reagent (redirect from Fenton reaction)
than a specific Fenton reaction. In the electro-Fenton process, hydrogen peroxide is produced in situ from the electrochemical reduction of oxygen. Fenton's...
16 KB (1,627 words) - 03:42, 10 October 2024
(2019-04-25). "Influence of Electrolyte Composition on the Electrochemical Reaction Mechanism of Bismuth Fluoride Electrode in Fluoride Shuttle Battery"...
22 KB (2,317 words) - 03:07, 27 October 2024
Oxidizing agent (redirect from Oxidation half reaction)
Electrosynthesis – Synthesis of chemical compounds in an electrochemical cell Organic oxidation – Redox reaction that takes place with organic compoundsPages displaying...
9 KB (875 words) - 16:12, 12 October 2024
luminescence produced during electrochemical reactions in solutions. In electrogenerated chemiluminescence, electrochemically generated intermediates undergo...
9 KB (922 words) - 09:51, 9 October 2024
Reductive elimination (category Reaction mechanisms)
Since oxidative addition and reductive elimination are reverse reactions, the same mechanisms apply for both processes, and the product equilibrium depends...
13 KB (1,441 words) - 22:05, 12 November 2024
Hoffman's work, which introduced an electrochemical method for exfoliation in 1938. The development of electrochemical exfoliation piqued the interest of...
37 KB (4,535 words) - 15:32, 22 August 2024
Chemical kinetics (redirect from Reaction kinetics)
conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction...
24 KB (3,326 words) - 19:09, 2 November 2024
process generates aptamers. Electrochemical aptamer-based (E-AB) biosensors is a device that takes advantage of the electrochemical and biological properties...
28 KB (3,457 words) - 15:02, 20 October 2024
Proton-coupled electron transfer (category Reaction mechanisms)
[(bipy)2(py)RuIV(O)]2+ + [(bipy)2(py)RuII(OH2)]2+ → 2 [(bipy)2(py)RuIII(OH)]2+ Electrochemical reactions where reduction is coupled to protonation or where oxidation is...
8 KB (895 words) - 20:42, 15 January 2024
Tafel equation (category Electrochemical equations)
The Tafel equation is an equation in electrochemical kinetics relating the rate of an electrochemical reaction to the overpotential. The Tafel equation...
10 KB (1,161 words) - 10:10, 14 November 2024