Electrophilic substitution of unsaturated silanes involves attack of an electrophile on an allyl- or vinylsilane. An allyl or vinyl group is incorporated...
12 KB (1,477 words) - 15:22, 7 February 2024
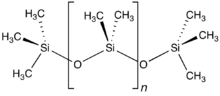
Organosilicon chemistry (redirect from Alkyl silane)
formal allylic substitution on the benzoyloxy group takes place. Unsaturated silanes like the above are susceptible to electrophilic substitution. Organosilicon...
25 KB (2,547 words) - 00:08, 6 April 2024
Silicon compounds (redirect from Compounds of silicon)
viable method of producing substituted silanes. The silanes comprise a homologous series of silicon hydrides with a general formula of Si nH 2n + 2. They...
42 KB (5,607 words) - 22:19, 30 May 2024
Ylide (section Dehydrocoupling with silanes)
condensation of an α-amino acid and an aldehyde or by thermal ring opening reaction of certain N-substituted aziridines. The further-unsaturated nitrile ylides...
10 KB (1,152 words) - 18:33, 21 August 2024
Sigma complex (section Types of sigma complexes)
the electrophilic substitution reaction on an aromatic compound. In the halogenation of benzene, the sigma complex comprises the six carbon atoms of the...
5 KB (506 words) - 18:38, 27 October 2024
(hetero) arenes, unactivated olefins and unsaturated carboxylic acids. The reaction mechanism of electrophilic trifluoromethylations has been described...
43 KB (4,098 words) - 21:26, 14 December 2024
activates the electrophilic carbon in presence of allyltrimethylsilane which then undergoes nucleophilic attack from electrons on the allylic silane. The silicon...
6 KB (580 words) - 22:33, 27 September 2024
Hydride (section Types of hydrides)
agents in chemical synthesis. The hydride adds to an electrophilic center, typically unsaturated carbon. Hydrides such as sodium hydride and potassium...
21 KB (2,300 words) - 08:43, 9 December 2024
Cycloalkane (section Table of cycloalkanes)
react in electrophilic addition, but in nucleophilic aliphatic substitution. These reactions are ring-opening reactions or ring-cleavage reactions of alkyl...
23 KB (2,397 words) - 08:22, 27 December 2024
sulfonates, silanes, and stannanes. However, some side reactions are commonly observed. Most side reactions occur due to the Lewis-acidity of the byproduct...
27 KB (2,906 words) - 19:24, 9 October 2024
Alkyne (category Pages that use a deprecated format of the chem tags)
{H}}{C}}}}{\ce {-H}}} 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest...
24 KB (3,174 words) - 20:24, 24 December 2024
reaction does not proceed without an alcoholic additive. Acyl silanes are less electrophilic than the corresponding aldehydes, preventing typical benzoin-type...
19 KB (2,357 words) - 19:34, 9 March 2023
Benzene (section Component of gasoline)
reactions of benzene involve substitution of a proton by other groups. Electrophilic aromatic substitution is a general method of derivatizing benzene. Benzene...
91 KB (9,589 words) - 07:53, 16 December 2024
Silyl enol ether (category Silanes)
increase the rate of reaction, trimethylsilyl triflate may also be used in the place of trimethylsilyl chloride as a more electrophilic substrate. When...
13 KB (1,369 words) - 19:53, 12 February 2024
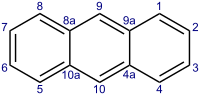
acetylacetonate. Electrophilic substitution of anthracene occurs at the 9 position. For example, formylation affords 9-anthracenecarboxaldehyde. Substitution at other...
17 KB (1,274 words) - 19:13, 18 December 2024
heterolytic cleavage of a carbon–halogen bond in an alkyl halide and generates a carbocation, which undergoes electrophilic aromatic substitution. Although vastly...
45 KB (5,026 words) - 04:16, 15 October 2024
K. In electrophilic aromatic substitution reactions, naphthalene reacts more readily than benzene. For example, chlorination and bromination of naphthalene...
37 KB (3,377 words) - 16:28, 9 December 2024
Carbon dioxide (redirect from Biological roles of carbon dioxide)
benzaldehyde or strongly electrophilic α,β-unsaturated carbonyl compounds. However, unlike electrophiles of similar reactivity, the reactions of nucleophiles with...
112 KB (12,757 words) - 00:27, 23 December 2024
Alkane (section Table of alkanes)
corresponding to loss of CH2 groups. Alkanes are only weakly reactive with most chemical compounds. They only reacts with the strongest of electrophilic reagents by...
62 KB (6,986 words) - 13:34, 25 December 2024