An enthalpy–entropy chart, also known as the H–S chart or Mollier diagram, plots the total heat against entropy, describing the enthalpy of a thermodynamic...
7 KB (804 words) - 13:54, 9 August 2024
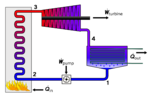
heat source and the working fluid and therefore reducing the amount of entropy generated by irreversibility. Rankine engines generally operate in a closed...
18 KB (2,301 words) - 16:10, 9 March 2024
particularly for water, steam, and moist air. Mollier diagrams (enthalpy-entropy charts) are routinely used by engineers in the design work associated...
3 KB (327 words) - 16:55, 5 October 2023
Thermodynamic databases for pure substances (redirect from Enthalpy of transition)
thermodynamic properties for substances, the most important being enthalpy, entropy, and Gibbs free energy. Numerical values of these thermodynamic properties...
26 KB (3,579 words) - 20:01, 7 July 2024
at points on the enthalpy-entropy and Mach number-entropy diagrams, which is meaningful for many applications. However, the entropy values for each model...
11 KB (1,900 words) - 15:12, 3 May 2024
Phase diagram (redirect from Thermodynamic chart)
constant value. Chart in U.S. units enthalpy–entropy (h–s) diagram for steam pressure–enthalpy (p–h) diagram for steam temperature–entropy (T–s) diagram...
22 KB (2,517 words) - 09:13, 15 June 2024
at points on the enthalpy-entropy and Mach number-entropy diagrams, which is meaningful for many applications. However, the entropy values for each model...
10 KB (1,778 words) - 15:13, 3 May 2024
thermodynamic quantity in the standard state, such as change in enthalpy (ΔH°), change in entropy (ΔS°), or change in Gibbs free energy (ΔG°). The degree symbol...
17 KB (2,067 words) - 09:01, 10 July 2024
variables are the internal energy U ( S , V ) {\displaystyle U(S,V)} , enthalpy H ( S , P ) {\displaystyle H(S,P)} , Helmholtz free energy F ( T , V )...
19 KB (3,124 words) - 07:31, 9 May 2024
evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Steam that is saturated or superheated (water vapor) is...
14 KB (1,552 words) - 22:31, 3 May 2024
Enthalpy Enthalpy change of solution Enthalpy of fusion Enthalpy of neutralization Enthalpy of sublimation Enthalpy of vaporization Enthalpy–entropy chart...
20 KB (2,038 words) - 06:17, 14 June 2024
{1}{V}}\left({\frac {\partial V}{\partial p}}\right)_{S},} where S is entropy. For a solid, the distinction between the two is usually negligible. Since...
13 KB (1,661 words) - 19:39, 15 May 2024
including the macroscopic entropy, though microscopically referable to the Gibbs statistical mechanical definition of entropy for the canonical ensemble...
104 KB (12,965 words) - 16:41, 3 August 2024
Van der Waals equation (section Enthalpy)
{\displaystyle c_{p}} have simple analytic expressions [this is also true of enthalpy h = u + p v {\displaystyle h=u+pv} , Helmholtz free energy f = u − T s...
81 KB (15,378 words) - 21:13, 23 July 2024
h3 is the specific enthalpy at state three h4 is the specific enthalpy at state 4 for the actual turbine h4s is the specific enthalpy at state 4s for the...
67 KB (9,414 words) - 15:56, 9 July 2024
doi:10.1016/0031-8914(48)90032-9. Properties of Natural Gases. Includes a chart of compressibility factors versus reduced pressure and reduced temperature...
6 KB (547 words) - 23:03, 18 July 2023
be related to its Gibbs free energy of formation, and the energy terms (enthalpy of formation) can be calculated given the defect concentration or vice...
11 KB (1,341 words) - 16:00, 15 June 2024
to determine exergy. For a given set of chemicals at a given entropy and pressure, enthalpy H is used in the expression: For a given set of chemicals at...
81 KB (11,002 words) - 23:27, 6 June 2024
ions) of the two substances, and of thermodynamic concepts such as enthalpy and entropy. Under certain conditions, the concentration of the solute can exceed...
50 KB (6,544 words) - 02:47, 3 July 2024
the original on 9 June 2007. Retrieved 4 June 2007. "Dielectric Constants Chart". ASI Instruments. Archived from the original on 29 May 2007. Retrieved...
11 KB (184 words) - 06:35, 19 January 2023
Compressibility factor (redirect from Compressibility chart)
factor for specific gases can be read from generalized compressibility charts that plot Z {\displaystyle Z} as a function of pressure at constant temperature...
23 KB (2,802 words) - 23:21, 19 December 2023
consequences of manipulating this material. For instance, a temperature–entropy diagram (T–s diagram) may be used to demonstrate the behavior of a fluid...
9 KB (1,235 words) - 19:26, 9 April 2024
kJ·mol−1 = 1000 joules per mole). Free energy is made up of an enthalpy term and an entropy term. Δ G ⊖ = Δ H ⊖ − T Δ S ⊖ {\displaystyle \Delta G^{\ominus...
103 KB (11,513 words) - 19:06, 17 June 2024
between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus...
26 KB (3,080 words) - 18:17, 27 May 2024
temperature) into functions of the conjugate quantity (momentum, volume, and entropy, respectively). In this way, it is commonly used in classical mechanics...
51 KB (8,922 words) - 15:44, 18 August 2024
flow of a perfect gas is assumed. Stagnation temperature and stagnation enthalpy are the same upstream and downstream of the shock. Normal shock waves can...
27 KB (3,811 words) - 08:14, 12 July 2024
due to the lower enthalpy of formation of iron(III) oxide (−824.2 kJ/mol) compared to sodium hydroxide (−500 kJ/mol) and positive entropy change of the reaction...
54 KB (5,892 words) - 18:16, 8 August 2024
Ethanol (data page) (section Charts)
(241 °C), 63 bar Std enthalpy change of fusion, ΔfusHo +4.9 kJ/mol Std entropy change of fusion, ΔfusSo +31 J/(mol·K) Std enthalpy change of vaporization...
24 KB (259 words) - 09:22, 23 July 2024
"Methyl Chloride". Inchem. Retrieved 30 May 2007. "Dielectric Constants Chart". ASI Instruments. Archived from the original on 29 May 2007. Retrieved...
8 KB (167 words) - 11:27, 25 March 2024
terms Mollier chart Enthalpy–enthalpy diagram, showing the enthalpy on the vertical axis, and the entropy on the horizontal axis This chart is useful for...
15 KB (60 words) - 06:38, 12 May 2024