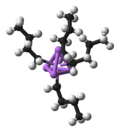
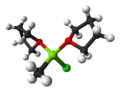
A Gilman reagent is a diorganocopper compound with the formula Li[CuR2], where R is an alkyl or aryl. They are colorless solids.[citation needed] These...
7 KB (696 words) - 13:57, 14 September 2024
father of organometallic chemistry. He discovered the Gilman reagent, which bears his name. Henry Gilman was born in Boston, Massachusetts, as the son of a...
10 KB (1,200 words) - 09:09, 29 September 2024
Organometallic chemistry (redirect from Organometallic reagent)
Examples of organometallic compounds include Gilman reagents, which contain lithium and copper, and Grignard reagents, which contain magnesium. Boron-containing...
31 KB (3,157 words) - 20:03, 24 September 2024
to: Turbo-Grignards, organocerium reagents, and organocuprate (Gilman) reagents. Turbo-Grignards are Grignard reagents modified with lithium chloride. Compared...
12 KB (1,095 words) - 14:22, 26 September 2024
Gilman may refer to: Gilman Ranch, California Gilman, Colorado Gilman, Illinois Gilman, Iowa Gilman, Minnesota Gilman, Montana Gilman, Vermont Gilman...
1 KB (159 words) - 23:38, 23 April 2023
second step, a lithium dialkylcuprate, also known as a Gilman reagent (named after Henry Gilman of Iowa State University) is prepared from the alkyllithium...
11 KB (1,505 words) - 20:21, 12 July 2024
often called Gilman reagents – can add to acid halides just once to give ketones. The reaction between an acid halide and a Gilman reagent is not a nucleophilic...
20 KB (2,351 words) - 23:32, 25 August 2024
(Grignard-Grisius-Cormas-Gilman) reaction scheme. In this aspect, they are similar to organolithium reagents. Grignard reagents are rarely isolated as solids...
24 KB (2,750 words) - 02:03, 18 October 2024
trifluoride etherate, these reagents are used for conjugate addition reactions. Lower-order cuprates (R2CuLi, also known as Gilman reagents) result when organocopper...
15 KB (1,732 words) - 05:45, 8 November 2023
laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric...
141 KB (13,803 words) - 10:12, 6 October 2024
Karl Ziegler, Georg Wittig, and Henry Gilman. In comparison with Grignard (magnesium) reagents, organolithium reagents can often perform the same reactions...
55 KB (5,971 words) - 20:35, 22 July 2024
Organocopper chemistry (redirect from Normant reagents)
conjugate addition of the Gilman reagent to an enone: In a so-called rapid-injection NMR experiment at −100 °C, the Gilman reagent Li+[Cu(CH3)2]− (stabilized...
23 KB (2,441 words) - 02:00, 1 August 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
40 KB (3,205 words) - 19:51, 17 October 2024
with Grignard reagents, terminal alkynes or organolithium reagents; in particular, the last reaction described produces a Gilman reagent. These can undergo...
122 KB (13,910 words) - 14:34, 15 October 2024
Cope elimination Cope rearrangement Corey reagent Corey–Bakshi–Shibata reduction Corey–Fuchs reaction Corey–Gilman–Ganem oxidation Corey–Kim oxidation Corey-Nicolaou...
37 KB (3,419 words) - 11:08, 19 October 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
22 KB (2,377 words) - 12:31, 8 August 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
1 KB (136 words) - 00:57, 7 June 2023
reaction Fluorination Formylation Fowler process Fukuyama coupling Gilman reagent coupling Glaser coupling Gomberg–Bachmann reaction Haber–Weiss reaction...
4 KB (296 words) - 16:47, 16 February 2024
cooling and reacting with various reagents in a sequence of hydrometalurgical processing steps. Suitable extraction reagents include alkali metal sulfates...
17 KB (1,469 words) - 15:36, 18 October 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
7 KB (480 words) - 18:59, 8 March 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
30 KB (2,641 words) - 00:48, 16 October 2024
PMC 3357580. PMID 22690368. Brunton L, Chabner B, Knollman B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill...
93 KB (9,984 words) - 07:20, 17 October 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
10 KB (980 words) - 18:03, 6 October 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
5 KB (270 words) - 18:07, 1 July 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
14 KB (1,096 words) - 12:21, 25 August 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
14 KB (1,012 words) - 04:57, 17 January 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
13 KB (1,424 words) - 17:26, 17 August 2024
Organic (soaps) Hemolithin (extraterrestrial protein) Organolithium reagents Gilman reagent CH3COOLi C4H6LiNO4 LiC2F6NO4S2 LiN(SiMe3)2 Li3C6H5O7 C5H5Li LiN(C3H7)2...
9 KB (725 words) - 18:53, 9 June 2024
Nagata reaction the cyanide source is diethylaluminum cyanide. The Gilman reagent is an effective nucleophile for 1,4-additions to conjugated carbonyls...
6 KB (625 words) - 20:20, 5 March 2024
Lithium diisopropylamide (category Reagents for organic chemistry)
and carboxylic acids are readily deprotonated. Like most organolithium reagents, LDA is not a salt, but is highly polar. It forms aggregates in solution...
8 KB (776 words) - 23:57, 16 May 2024