In chemistry, a hydrogen bond (or H-bond) is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a...
46 KB (5,463 words) - 19:48, 6 November 2024
the carbon–hydrogen bond (C−H bond) is a chemical bond between carbon and hydrogen atoms that can be found in many organic compounds. This bond is a covalent...
6 KB (533 words) - 20:36, 1 October 2024
Intermolecular force (redirect from Dipole-dipole bond)
categorized into the following types: Hydrogen bonding Ion–dipole forces and ion–induced dipole force Cation–π, σ–π and π–π bonding Van der Waals forces – Keesom...
28 KB (3,476 words) - 01:45, 21 December 2024
Hydrogen-bond catalysis is a type of organocatalysis that relies on use of hydrogen bonding interactions to accelerate and control organic reactions....
33 KB (3,990 words) - 23:54, 6 December 2024
such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding. Since opposite electric charges attract, the negatively charged electrons...
40 KB (4,872 words) - 13:33, 22 September 2024
extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen halides. Hydrogen fluoride is...
15 KB (1,376 words) - 00:57, 7 December 2024
carbon–hydrogen bond activation (C−H activation) is a type of organic reaction in which a carbon–hydrogen bond is cleaved and replaced with a C−X bond (X...
36 KB (3,907 words) - 16:18, 23 November 2024
hydrogen bond is a special type of hydrogen bond in which the proton is spaced exactly halfway between two identical atoms. The strength of the bond to...
2 KB (187 words) - 13:26, 22 September 2024
Non-covalent interaction (redirect from Non-covalent bond)
(Na+). A hydrogen bond (H-bond), is a specific type of interaction that involves dipole–dipole attraction between a partially positive hydrogen atom and...
27 KB (3,311 words) - 07:44, 12 November 2024
Amide (redirect from Amide bond)
groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs...
24 KB (2,303 words) - 02:33, 14 December 2024
"co-valent bond", in essence, means that the atoms share "valence", such as is discussed in valence bond theory. In the molecule H 2, the hydrogen atoms share...
28 KB (3,673 words) - 16:31, 3 December 2024
Non-canonical base pairs are planar hydrogen bonded pairs of nucleobases, having hydrogen bonding patterns which differ from the patterns observed in...
89 KB (11,719 words) - 14:07, 29 July 2024
Salicylaldehyde (section Internal hydrogen bonding)
internal hydrogen bond is formed between the groups. The hydroxy group serves here as the hydrogen bond donor, and the aldehyde as hydrogen bond acceptor...
9 KB (774 words) - 13:03, 21 October 2024
Beta turn (section Hydrogen bond criterion)
can be defined in two ways: By the possession of an intra-main-chain hydrogen bond between the CO of residue i and the NH of residue i+3; By having a distance...
9 KB (1,100 words) - 03:01, 2 March 2024
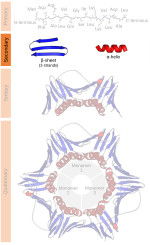
Beta sheet (section Hydrogen bonding patterns)
proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands...
27 KB (3,120 words) - 01:54, 14 August 2024
molecule, the bond-dissociation energy of an oxygen–hydrogen bond varies slightly depending on whether or not there is another hydrogen atom bonded to...
9 KB (1,318 words) - 02:44, 29 April 2024
Alpha helix (section Geometry and hydrogen bonding)
with 13 atoms being involved in the ring formed by the hydrogen bond (starting with amidic hydrogen and ending with carbonyl oxygen) In the early 1930s,...
46 KB (5,219 words) - 12:42, 20 November 2024
mechanics through the exploration of its energetics and chemical bonding. Hydrogen gas was first produced artificially in the early 16th century by reacting...
121 KB (12,377 words) - 14:27, 16 September 2024
Properties of water (redirect from Hydrogen Hydroxide)
dissociate ions in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties...
89 KB (9,582 words) - 15:05, 19 December 2024
Atoms in molecules (redirect from Hydrogen hydrogen bond)
two ortho hydrogen atoms again is shorter than their van der Waals radii and according to in silico experiments based on this theory, a bond path is identified...
12 KB (1,525 words) - 14:29, 25 August 2024
oxidizer in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes...
92 KB (9,247 words) - 08:35, 19 December 2024
placing the peptide group in a hydrophobic environment or donating a hydrogen bond to the nitrogen atom of an X-Pro peptide group. Both of these mechanisms...
12 KB (1,388 words) - 16:38, 18 December 2024
formula HCl. Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine...
27 KB (2,812 words) - 17:06, 25 October 2024
Chemical polarity (redirect from Polar bond)
polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole-dipole intermolecular forces and hydrogen bonds. Polarity...
24 KB (2,751 words) - 01:53, 11 December 2024
Base pair (section Hydrogen bonding and stability)
to the folded structure of both DNA and RNA. Dictated by specific hydrogen bonding patterns, "Watson–Crick" (or "Watson–Crick–Franklin") base pairs (guanine–cytosine...
32 KB (3,701 words) - 14:01, 27 November 2024
Orbital hybridisation (redirect from Sp² bond)
two bonds is the tetrahedral bond angle of 109°28' (around 109.5°). Pauling supposed that in the presence of four hydrogen atoms, the s and p orbitals...
33 KB (3,185 words) - 20:06, 22 December 2024
The parent components of deep eutectic solvents engage in a complex hydrogen bonding network, which results in significant freezing point depression as...
22 KB (2,553 words) - 21:21, 31 October 2024
Ketone (section Structure and bonding)
are not usually hydrogen-bond donors and cannot hydrogen-bond to themselves. Because of their inability to serve both as hydrogen-bond donors and acceptors...
24 KB (2,895 words) - 03:58, 25 November 2024
A Low-barrier hydrogen bond (LBHB) is a special type of hydrogen bond. LBHBs can occur when the pKa of the two heteroatoms are closely matched, which...
6 KB (687 words) - 13:38, 21 December 2024
helix and π helix, are calculated to have energetically favorable hydrogen-bonding patterns but are rarely observed in natural proteins except at the...
28 KB (3,076 words) - 03:33, 27 October 2024