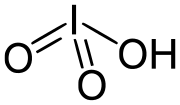
Iodine oxides are chemical compounds of oxygen and iodine. Iodine has only two stable oxides which are isolatable in bulk, iodine tetroxide and iodine...
8 KB (738 words) - 16:42, 4 August 2024
Gay-Lussac, after the Ancient Greek Ιώδης, meaning 'violet'. Iodine occurs in many oxidation states, including iodide (I−), iodate (IO− 3), and the various...
107 KB (12,026 words) - 02:11, 19 October 2024
Iodine pentoxide is the chemical compound with the formula I2O5. This iodine oxide is the anhydride of iodic acid, and one of the few iodine oxides that...
4 KB (264 words) - 19:27, 2 August 2024
the simplest of many iodine oxides. It is similar to the oxygen monofluoride, chlorine monoxide and bromine monoxide radicals. Iodine monoxide can be obtained...
4 KB (271 words) - 12:28, 4 January 2024
bromine monoxide radical: Bromine oxide (BrO) Oxygen fluoride Chlorine oxide Iodine oxide This set index article lists chemical compounds articles associated...
877 bytes (85 words) - 16:43, 11 May 2023
Iodine compounds are compounds containing the element iodine. Iodine can form compounds using multiple oxidation states. Iodine is quite reactive, but...
30 KB (3,558 words) - 02:08, 12 September 2024
Iodine dioxide is a binary inorganic compound of iodine and oxygen with the chemical formula IO 2. This compound is one of many iodine oxides. The compound...
4 KB (272 words) - 22:29, 1 August 2024
ClO−4 Oxygen fluoride(s), bromine oxide(s), iodine oxide(s) – analogous oxygen halide and halogen oxides Sulfur fluoride(s), sulfur chloride(s), sulfur...
2 KB (286 words) - 00:10, 30 November 2023
Diiodine oxide, also known as iodo hypoiodite, is an oxide of iodine that is equivalent to an acid anhydride of hypoiodous acid. This substance is unstable...
2 KB (108 words) - 06:15, 29 September 2024
Tetraiodine nonoxide (category Iodine compounds)
Tetraiodine nonoxide is an iodine oxide with the chemical formula I4O9. Tetraiodine nonoxide can be produced by reacting ozone and iodine in carbon tetrachloride...
2 KB (233 words) - 19:25, 28 February 2024
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered...
7 KB (583 words) - 04:40, 16 May 2024
Tungsten(IV) oxide is the chemical compound with the formula WO2. The bronze-colored solid crystallizes in a monoclinic cell. The rutile-like structure...
3 KB (210 words) - 11:26, 1 March 2024
Oxygen fluoride (redirect from Fluorine oxide)
reactivity is also dependent on the type of fuel used. Bromine oxide Chlorine oxide Iodine oxide Ozone Solomon, I. J.; et al. (1968). "Additional Studies Concerning...
9 KB (959 words) - 07:34, 10 February 2024
In chemistry, the iodine value (IV; also iodine absorption value, iodine number or iodine index) is the mass of iodine in grams that is consumed by 100 grams...
25 KB (2,513 words) - 20:38, 19 September 2024
Phenol oxidation with hypervalent iodine reagents leads to the formation of quinone-type products or iodonium ylides, depending on the structure of the...
9 KB (1,091 words) - 03:35, 7 May 2022
Bromine (section Bromine oxides and oxoacids)
it is analogous to triiodide. Bromine oxides are not as well-characterised as chlorine oxides or iodine oxides, as they are all fairly unstable: it was...
67 KB (7,715 words) - 20:33, 10 August 2024
Iodic acid (category Iodine compounds)
features iodine in the oxidation state +5 and is one of the most stable oxo-acids of the halogens. When heated, samples dehydrate to give iodine pentoxide...
8 KB (644 words) - 02:04, 12 September 2024
Diiodine tetroxide (redirect from Iodine tetroxide)
and iodine. It belongs to the class of iodine oxides, and is a mixed oxide, consisting of iodine(III) and iodine(V) oxidation states. The oxide is formed...
4 KB (393 words) - 02:07, 7 September 2024
Povidone-iodine (PVP-I), also known as iodopovidone, is an antiseptic used for skin disinfection before and after surgery. It may be used both to disinfect...
22 KB (2,243 words) - 21:48, 21 September 2024
REACTIONS IO + NO2 + M → IONO2 + M, IO + IO → PRODUCTS, AND ATOMIC IODINE + OZONE → IODINE OXIDE (IO) + MOLECULAR OXYGEN". Chemischer Informationsdienst. 16...
2 KB (150 words) - 16:26, 6 February 2024
Iodite (category Iodine oxyanions)
iodite ion, or iodine dioxide anion, is the halite with the chemical formula IO− 2. Within the ion, the iodine exists in the oxidation state of +3. Iodites...
2 KB (207 words) - 18:24, 31 August 2024
Carbonyl oxidation with hypervalent iodine reagents involves the functionalization of the α position of carbonyl compounds through the intermediacy of...
10 KB (1,207 words) - 14:57, 28 August 2023
Potassium iodide (redirect from Potassium Iodine)
measure to prevent iodine deficiency in populations that get little seafood). The oxidation of iodide causes slow loss of iodine content from iodised...
54 KB (5,663 words) - 04:43, 29 June 2024
Periodate (section Oxidation reactions)
composed of iodine and oxygen. It is one of a number of oxyanions of iodine and is the highest in the series, with iodine existing in oxidation state +7...
19 KB (2,111 words) - 17:56, 31 August 2024
(/ˌpɜːraɪˈɒdɪk/ per-eye-OD-ik) is the highest oxoacid of iodine, in which the iodine exists in oxidation state +7. It can exist in two forms: orthoperiodic...
12 KB (976 words) - 07:10, 1 September 2024
The sulfur–iodine cycle (S–I cycle) is a three-step thermochemical cycle used to produce hydrogen. The S–I cycle consists of three chemical reactions whose...
10 KB (1,081 words) - 22:03, 7 April 2024
Iodous acid (category Iodine compounds)
disproportionate to molecular iodine and iodates. Iodous acid is part of a series of oxyacids in which iodine can assume oxidation states of −1, +1, +3, +5...
2 KB (90 words) - 18:22, 31 August 2024
Iodosyl trifluoride (redirect from Iodine oxide trifluoride)
Jimmy W.; Baird, H. Wallace (1 January 1967). "The crystal structure of iodine oxide trifluoride". Chemical Communications (21): 1093–1094. doi:10.1039/C19670001093...
3 KB (237 words) - 14:29, 19 June 2023
greater sensitivity to water. Selte, Kari; Kjekshus, Arne (1971). "Iodine Oxides. Part IV. Solid Compounds Formed in the Systems H2O—SO3—I2On (n=3, 4...
5 KB (409 words) - 04:56, 16 October 2024
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California...
38 KB (4,377 words) - 14:26, 12 September 2024