Iodometry, known as iodometric titration, is a method of volumetric chemical analysis, a redox titration where the appearance or disappearance of elementary...
9 KB (1,149 words) - 10:54, 10 May 2024
interaction between starch and the triiodide anion (I− 3) is the basis for iodometry. The iodine–starch test was first described in 1814 by Jean-Jacques Colin...
12 KB (1,287 words) - 02:10, 12 September 2024
Sodium thiosulfate (section Iodometry)
Na2S2O3·5H2O has an excellent shelf-life, it is used as a titrant in iodometry. Na2S2O3·5H2O is also a component of iodine clock experiments. This particular...
14 KB (1,293 words) - 06:37, 8 September 2024
Proceedings of the Indian National Science Academy. 46 (6). Indian National Science Academy: 562–564. ISSN 0370-0046. Retrieved 2009-03-20. Iodometry v t e...
802 bytes (66 words) - 01:29, 16 July 2024
room temperature. Like iodine monochloride, IBr is used in some types of iodometry. It serves as a source of I+. Its Lewis acid properties are compared with...
3 KB (187 words) - 04:29, 28 December 2023
Redox titration Titrant Iodometry Iodine (I2) Bromatometry Bromine (Br2) Cerimetry Cerium(IV) salts Permanganometry Potassium permanganate Dichrometry...
2 KB (227 words) - 02:22, 6 October 2024
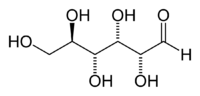
Glucose (section Copper iodometry)
(fluorescence-labeled) enzymes as reporters. Glucose can be quantified by copper iodometry. In particular, for the analysis of complex mixtures containing glucose...
121 KB (12,789 words) - 20:45, 27 October 2024
Tribromide Polyhalogen ions Three-center four-electron bond Iodine–starch test Iodometry Povidone-iodine Lugol's iodine Dye-sensitized solar cell Organic superconductor...
16 KB (1,587 words) - 04:56, 16 October 2024
anions can be used for quantitative volumetric analysis, for example in iodometry. Iodine and starch form a blue complex, and this reaction is often used...
104 KB (11,657 words) - 18:48, 27 October 2024
oxidizing agent, and several procedures have been developed. The popular iodometry approach uses iodine in the presence of a starch indicator. Iodine is...
24 KB (2,359 words) - 19:48, 27 October 2024
tetrathionate ions: 2 S2O2−3 + I2 → S4O2−6 + 2 I− This reaction is key for iodometry. With bromine (X = Br) and chlorine (X = Cl), thiosulfate ions are oxidized...
14 KB (1,408 words) - 22:15, 29 October 2024
paints. The determination of iodine value is a particular example of iodometry. A solution of iodine I2 is yellow/brown in color. When this is added...
25 KB (2,513 words) - 13:14, 21 October 2024
quantitatively, the bromine consumed by a sample can be determined by iodometry. The bromine number indicates the degree of unsaturation of a sample....
2 KB (325 words) - 19:09, 1 June 2023
of the color of the excess oxidizing agent potassium permanganate. In iodometry, at sufficiently large concentrations, the disappearance of the deep red-brown...
39 KB (4,669 words) - 00:36, 24 October 2024
cylindrical stir bar can be used for suspension of solids, as seen in iodometry, deagglomeration (useful for preparation of microbiology growth medium...
39 KB (4,968 words) - 13:56, 28 October 2024
oxidation states suitable for cerimetry is determined in one titration. Iodometry Redox titration Nonstoichiometry Redox indicator Oxidizing agent Reducing...
4 KB (435 words) - 17:29, 21 February 2021
obtained by oxidation of thiosulfate ion with iodine (reaction is used in iodometry): S2O2−3 + I2 → S4O2−6 + 2 I− More specialized routes involve reactions...
2 KB (273 words) - 04:29, 28 December 2023
Tutundžić gave original pioneering contributions to the development of iodometry, metalometry, permanganometry, bichromatometry, indirect coulometric titration...
6 KB (615 words) - 07:12, 24 August 2024