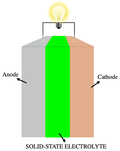
Ionic conductivity (denoted by λ) is a measure of a substance's tendency towards ionic conduction. Ionic conduction is the movement of ions. The phenomenon...
3 KB (369 words) - 23:21, 2 July 2024
current Conductivity (electrolytic), the electrical conductivity of an electrolyte in solution Ionic conductivity (solid state), electrical conductivity due...
651 bytes (111 words) - 17:40, 29 September 2023
Ionic conductivity may refer to: Conductivity (electrolytic), electrical conductivity due to an electrolyte separating into ions in solution Ionic conductivity...
311 bytes (68 words) - 13:16, 22 August 2024
solid-state electrolyte (SSE) is a solid ionic conductor and electron-insulating material and it is the characteristic component of the solid-state battery...
53 KB (5,967 words) - 12:12, 23 August 2024
A solid-state battery (SSB) is an electrical battery that uses a solid electrolyte for ionic conductions between the electrodes, instead of the liquid...
90 KB (9,514 words) - 14:22, 19 December 2024
Solid-state ionics is the study of ionic-electronic mixed conductor and fully ionic conductors (solid electrolytes) and their uses. Some materials that...
26 KB (3,262 words) - 13:48, 3 June 2024
electrolyte thickness, and σ {\displaystyle \sigma } – ionic conductivity. The ionic conductivity of the solid oxide is defined as follows: σ = σ 0 ⋅ e − E R...
70 KB (9,220 words) - 18:59, 18 December 2024
and reliable way of measuring the ionic content in a solution. For example, the measurement of product conductivity is a typical way to monitor and continuously...
30 KB (3,493 words) - 02:21, 23 April 2024
electrolyte. Solid-state electrolytes are solids with high ionic conductivity, comparable to those of molten salts. Solid-state electrolytes have applications in...
23 KB (3,109 words) - 18:03, 26 November 2024
Network covalent bonding (redirect from Covalent network solid)
like those for ionic compounds, are simple ratios of the component atoms represented by a formula unit. Examples of network solids include diamond with...
4 KB (456 words) - 09:41, 4 December 2024
Salt (chemistry) (redirect from Ionic solid)
source of most transport phenomena within an ionic crystal, including diffusion and solid state ionic conductivity. When vacancies collide with interstitials...
63 KB (6,938 words) - 06:15, 8 December 2024
suited for application as solid electrolyte in solid oxide fuel cells. For low dopant concentrations, the ionic conductivity of the stabilized zirconias...
13 KB (1,446 words) - 15:03, 20 October 2024
Fluoride battery (section Solid electrolytes)
reactivity of naked fluoride in liquid electrolytes, low fluoride ionic conductivity of solid-state electrolytes at room temperature, and volume expansion of...
22 KB (2,317 words) - 22:57, 2 December 2024
Solid-state physics is the study of rigid matter, or solids, through methods such as solid-state chemistry, quantum mechanics, crystallography, electromagnetism...
10 KB (1,164 words) - 21:24, 19 November 2024
Fast-ion conductor (redirect from Solid electrolyte)
(glasses or crystals), the ionic conductivity σi can be any value, but it should be much larger than the electronic one. Usually, solids where σi is on the order...
12 KB (1,331 words) - 14:11, 14 July 2024
covalent solids (sometimes called simply "covalent solids") Ionic bonding, which forms ionic solids Metallic bonding, which forms metallic solids Weak inter...
11 KB (1,375 words) - 22:41, 20 December 2024
between the atoms in a solid can take a variety of forms. For example, a crystal of sodium chloride (common salt) is made up of ionic sodium and chlorine...
40 KB (5,369 words) - 14:55, 19 November 2024
physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid,...
35 KB (4,382 words) - 19:57, 23 August 2024
Conversely, when an ionic liquid is cooled, it often forms an ionic solid—which may be either crystalline or glassy. The ionic bond is usually stronger...
43 KB (4,706 words) - 07:07, 9 August 2024
together other solids: metallic (metallic bonding, 400–500 kJ mol−1), ionic (Coulomb’s forces, 700–900 kJ mol−1), and network solids (covalent bonds...
33 KB (2,941 words) - 16:03, 25 August 2024
Electrolyte (redirect from Ionic solution)
Brønsted base and in essence are protic ionic liquids in the molten state, have found to be promising solid-state proton conductors for fuel cells. Examples...
33 KB (3,443 words) - 00:25, 17 December 2024
investigation for its use in solid-state electrolytes in lithium-based battery technologies. LLZO has a high ionic conductivity and thermal and chemical stability...
8 KB (873 words) - 18:22, 27 April 2024
(LSCMS) is also being researched as a cathode material due to its high ionic conductivity. However, the rare-earth element introduces a significant materials...
24 KB (3,012 words) - 00:38, 10 December 2024
Lithium aluminium germanium phosphate (category Solid-state batteries)
and has been applied as a solid electrolyte in all-solid-state lithium-ion batteries. Typical values of ionic conductivity in LAGP at room temperature...
45 KB (4,916 words) - 02:39, 2 October 2024
Crystal (redirect from Morphous solid)
to each other. In general, solids can be held together by various types of chemical bonds, such as metallic bonds, ionic bonds, covalent bonds, van der...
33 KB (3,736 words) - 17:12, 27 November 2024
sometimes used. The SI unit of electrical conductivity is siemens per metre (S/m). Resistivity and conductivity are intensive properties of materials, giving...
75 KB (7,984 words) - 07:45, 6 December 2024
NASICON is an acronym for sodium (Na) super ionic conductor, which usually refers to a family of solids with the chemical formula Na1+xZr2SixP3−xO12,...
25 KB (2,814 words) - 13:51, 11 April 2024
Valence electron (section Electrical conductivity)
considered. Metallic elements generally have high electrical conductivity when in the solid state. In each row of the periodic table, the metals occur to the...
24 KB (2,333 words) - 15:26, 27 November 2024
the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the...
47 KB (11,453 words) - 13:41, 18 December 2024
high proton conductivity values of 2.2×10−2 S cm−1 at 240°C, and CsHSO4 boasts a proton conductivity of 4 × 10−2 S cm−1 at 200°C. The solid acid "superprotonic"...
26 KB (3,136 words) - 20:10, 9 November 2024