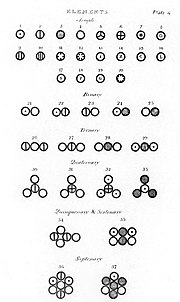
In chemistry, the law of definite proportions, sometimes called Proust's law or the law of constant composition, states that a given chemical compound...
7 KB (947 words) - 19:47, 9 October 2024
multiples of a basic quantity. Along with the law of definite proportions, the law of multiple proportions forms the basis of stoichiometry. The law of multiple...
9 KB (1,331 words) - 16:13, 11 September 2024
The law of reciprocal proportions, also called law of equivalent proportions or law of permanent ratios, is one of the basic laws of stoichiometry. It...
4 KB (531 words) - 01:05, 27 May 2024
Whole number rule (section Law of definite proportions)
whole-number rule." The law of definite proportions was formulated by Joseph Proust around 1800 and states that all samples of a chemical compound will have...
7 KB (847 words) - 18:49, 31 October 2023
Jöns Jacob Berzelius (category Members of the French Academy of Sciences)
reactions and that these occur in definite proportions. This understanding came to be known as the "Law of Constant Proportions". Berzelius was a strict empiricist...
43 KB (4,355 words) - 14:09, 29 September 2024
Jeremias Benjamin Richter (category Chemists from the Kingdom of Prussia)
existence of atoms was inferred from the law of definite proportions proposed by him in 1792.[dubious – discuss] Richter found that the ratio by weight of the...
6 KB (661 words) - 14:23, 20 August 2024
energy of such systems. Charge conservation Conservation law Fick's laws of diffusion Law of definite proportions Law of multiple proportions Sterner...
26 KB (3,411 words) - 22:59, 17 October 2024
Joseph Proust (category Members of the French Academy of Sciences)
for his discovery of the law of definite proportions in 1794, stating that chemical compounds always combine in constant proportions. Joseph-Louis Proust...
12 KB (1,161 words) - 16:16, 27 June 2024
Joseph Proust (1754–1826), French chemist, responsible for the Law of definite proportions Antonin Proust (1832–1905), French journalist and politician...
891 bytes (132 words) - 23:43, 24 September 2020
substances involved were transformed. Finally, there was the law of definite proportions, established by the French chemist Joseph Proust in 1797, which...
71 KB (9,314 words) - 02:32, 27 October 2024
Chemical substance (section Law)
Pearson Prentice Hall, Upper Saddle River, New Jersey, 2005. Law of Definite Proportions Archived November 18, 2007, at the Wayback Machine Hill, J. W...
26 KB (3,282 words) - 11:20, 25 October 2024
the Royal College, formulated the Law of Definite Proportions, a fundamental advance in modern chemistry. The Alcázar of Segovia has made its mark on cinema...
30 KB (3,542 words) - 16:07, 21 October 2024
Al-Jildaki (category Wikipedia articles incorporating text from the United States National Library of Medicine)
travels. He laid the basic building block for the creation of the "Law of definite Proportions" in chemical union, and explained it in detail, which Kepler...
21 KB (2,714 words) - 08:51, 26 July 2024
Avogadro constant (category Amount of substance)
the mass of one molecule relative to the mass of the hydrogen atom; which, because of the law of definite proportions, was the natural unit of atomic mass...
20 KB (2,232 words) - 23:10, 23 October 2024
Claude Louis Berthollet (category Members of the French Academy of Sciences)
on the validity of the law of definite proportions. While Proust believed that chemical compounds are composed of a fixed ratio of their constituent...
13 KB (1,231 words) - 12:10, 9 September 2024
Along with the law of multiple proportions, the law of definite proportions forms the basis of stoichiometry. The law of definite proportions and constant...
156 KB (19,387 words) - 17:47, 19 October 2024
Mole (unit) (redirect from Mole (unit of measurement))
units of continuous quantities In chemistry, it has been known since Proust's law of definite proportions (1794) that knowledge of the mass of each of the...
26 KB (3,294 words) - 05:24, 27 October 2024
to the mass of oxygen in the base. 1794: Proust's Law of definite proportions generalizes the concept of equivalent weights to all types of chemical reaction...
29 KB (3,578 words) - 05:10, 28 October 2024
law of definite composition says that pure chemicals are composed of elements in a definite formulation. Dalton's law of multiple proportions says that...
3 KB (420 words) - 07:48, 18 December 2023
Stoichiometry (redirect from Extent of reaction (chemistry))
conservation of mass, the law of definite proportions (i.e., the law of constant composition), the law of multiple proportions and the law of reciprocal...
35 KB (5,274 words) - 20:42, 28 October 2024
the atomic theory of John Dalton, Joseph Proust had developed the law of definite proportions, which later resulted in the concepts of stoichiometry and...
66 KB (8,043 words) - 01:08, 14 October 2024
Group 0 in his arrangement of the elements. As Mendeleev was doubtful of atomic theory to explain the law of definite proportions, he had no a priori reason...
15 KB (1,620 words) - 10:06, 13 August 2024
Equivalent weight (category Amount of substance)
theory. Equivalent weights were a useful generalisation of Joseph Proust's law of definite proportions (1794) which enabled chemistry to become a quantitative...
19 KB (2,706 words) - 02:54, 13 October 2024
Dalton (unit) (category Units of chemical measurement)
interpretation of the law of definite proportions in terms of the atomic theory of matter implied that the masses of atoms of various elements had definite ratios...
35 KB (3,683 words) - 18:19, 15 October 2024
diamond is a pure form of carbon. Louis Nicolas Vauquelin discovers chromium. Joseph Proust proposes the law of definite proportions, which states that elements...
6 KB (492 words) - 16:50, 16 June 2024
theories of the composition of substances. Although Dalton "won" for the most part, it was later recognized that the law of definite proportions had important...
17 KB (1,474 words) - 23:01, 6 February 2024
"1 cu mm of oil can cover 1 sq m of water." The law of definite proportions is stated in plain English (p. 123) as a "fundamental law of chemistry"...
13 KB (1,718 words) - 23:50, 4 May 2024
(1754–1826), discovered the Law of definite proportions Evgenii Przhevalsky (1879-1953), Russian and Soviet chemist, father of analytical chemistry in USSR...
61 KB (7,579 words) - 22:40, 15 October 2024
fraction. The law of definite composition and the law of multiple proportions are the first two of the three laws of stoichiometry, the proportions by which...
57 KB (5,649 words) - 14:21, 9 October 2024
quantitative chemical analysis. 1797 Joseph Proust proposes the law of definite proportions, which states that elements always combine in small, whole number...
72 KB (7,490 words) - 05:50, 11 September 2024