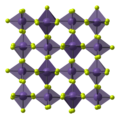
Manganese tetrafluoride, MnF4, is the highest fluoride of manganese. It is a powerful oxidizing agent and is used as a means of purifying elemental fluorine...
17 KB (1,598 words) - 21:58, 8 June 2024
Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting...
10 KB (778 words) - 18:22, 22 June 2024
Manganese fluoride can refer to: Manganese(II) fluoride, MnF2 Manganese(III) fluoride, MnF3 Manganese(IV) fluoride, MnF4 These three compounds are the...
380 bytes (88 words) - 15:02, 5 April 2024
Manganese(II) fluoride is the chemical compound composed of manganese and fluoride with the formula MnF2. It is a light pink solid, the light pink color...
5 KB (276 words) - 20:53, 3 January 2024
Manganese(IV) fluoride – MnF4 Manganese(IV) oxide (manganese dioxide) – MnO2 Manganese(II,III) oxide – Mn3O4 Manganese dioxide – MnO2 Manganese heptoxide...
119 KB (8,721 words) - 20:49, 6 December 2024
Manganese trioxide fluoride is an inorganic compound with the formula MnO3F. A green diamagnetic liquid, the compound has no applications, but it is of...
3 KB (257 words) - 02:11, 7 November 2024
Manganese is a chemical element; it has symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with...
86 KB (9,778 words) - 17:31, 31 December 2024
metal complexes with high spin d5 configurations. Manganese chloride is produced by treating manganese(IV) oxide with concentrated hydrochloric acid. MnO2...
10 KB (712 words) - 15:51, 8 October 2024
MnF3 manganese(III) fluoride 7783–53–1 MnF4 manganese(IV) fluoride 15195–58–1 MnI2 manganese(II) iodide 7790–33–2 MnI2•4H2O manganese(II) iodide tetrahydrate...
139 KB (120 words) - 17:07, 15 July 2024
oxide, silicon(IV) oxide, chromium(III) oxide, manganese(IV) oxide, iron(III) oxide, iron(II,III) oxide, copper(II) oxide, and lead(II,IV) oxide. In a thermochemical...
50 KB (5,823 words) - 06:48, 28 December 2024
Manganese(II) oxide is an inorganic compound with chemical formula MnO. It forms green crystals. The compound is produced on a large scale as a component...
6 KB (546 words) - 09:55, 26 May 2024
generally accepted trace elements are iron, chlorine, cobalt, copper, zinc, manganese, molybdenum, iodine, selenium, and bromine; there is some evidence that...
35 KB (3,068 words) - 19:41, 30 December 2024
Rhenium tetrafluoride (redirect from Rhenium(IV) fluoride)
Kemmitt, R. D. W.; Peacock, R. D. (26 January 2016). The Chemistry of Manganese, Technetium and Rhenium: Pergamon Texts in Inorganic Chemistry. Elsevier...
3 KB (169 words) - 15:30, 3 January 2024
K2ReF6. The compound can be prepared by reacting K2ReI6 with potassium fluoride, hydrogen iodide mixture. Also it can be formed by reacting K2ReI6 or K2ReCl6...
4 KB (243 words) - 21:38, 29 August 2024
purpose. The reaction of manganese dioxide with hydrochloric acid in tetrahydrofuran gives MnCl3(H2O)(THF)2. Manganese(III) fluoride suspended in THF reacts...
13 KB (1,088 words) - 05:28, 30 December 2024
Lithium-ion battery (redirect from Lithium-nickel-manganese-cobalt)
loss of manganese through disproportionation of a surface trivalent manganese to form a tetravalent manganese and a soluble divalent manganese: 2Mn3+ →...
207 KB (21,913 words) - 04:55, 30 December 2024
catalyst and as fertilizer. Manganese(II) acetate can be formed by treating either manganese(II,III) oxide or manganese(II) carbonate with acetic acid:...
5 KB (225 words) - 19:02, 7 November 2023
Fluorine (section Fluoride ion)
such as atorvastatin and fluoxetine contain C−F bonds. The fluoride ion from dissolved fluoride salts inhibits dental cavities and so finds use in toothpaste...
157 KB (15,331 words) - 17:27, 31 December 2024
AmF3(v)}}} The tetravalent americium(IV) fluoride (AmF4) is obtained by reacting solid americium(III) fluoride with molecular fluorine: 2 AmF 3 + F 2...
77 KB (9,345 words) - 00:28, 23 December 2024
suggested that Scheele analyze the properties of manganese(IV) oxide. It was through his studies of manganese(IV) oxide that Scheele developed his concept of...
32 KB (3,540 words) - 23:27, 23 September 2024
Nickel(III) fluoride is the chemical compound with the formula NiF3. It is an ionic compound of nickel and fluorine. Nickel(III) fluoride can be prepared...
2 KB (68 words) - 00:31, 29 March 2024
Trifluoride (category Fluorides)
trifluoride, NF3, a colorless, toxic, odourless, nonflammable gas Palladium(II,IV) fluoride, Pd[PF6], empirical formula PdF3 Phosphorus trifluoride, PF3, a colorless...
4 KB (372 words) - 07:56, 17 January 2024
soluble in water. When heated, the compound decomposes to the black nitride fluoride, ReNF. Donnay, Joseph Désiré Hubert (1963). Crystal Data; Determinative...
3 KB (176 words) - 14:11, 19 September 2024
Nitrogen pentafluoride (redirect from Perfluoroammonium fluoride)
bis(tetrafluoroammonium) hexafluoronickelate(IV) ([NF4]+)2[NiF6]2−. He also prepared compounds with manganese, a fluorouranate, tetrafluoroammonium perchlorate...
9 KB (827 words) - 13:17, 22 July 2024
structure found in tungsten, and the extremely complicated structure of manganese. Compounds that consist of more than one element (e.g. binary compounds)...
49 KB (3,620 words) - 14:56, 4 October 2024
in Li-ion batteries. Tin(II) fluoride is added to some dental care products as stannous fluoride (SnF2). Tin(II) fluoride can be mixed with calcium abrasives...
80 KB (8,979 words) - 10:35, 15 December 2024
Manganese(II) bromide is the chemical compound composed of manganese and bromine with the formula MnBr2. It can be used in place of palladium in the Stille...
3 KB (76 words) - 05:53, 30 August 2024
Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by...
10 KB (896 words) - 01:04, 5 August 2023
−596.3 Magnesium sulfate Solid MgSO4 −1170.6 Manganese Manganese(II) oxide Solid MnO −362.9 Manganese(IV) oxide Solid MnO2 −465.2 Mercury Mercury(II)...
9 KB (201 words) - 07:30, 9 April 2024
lattice energy of lithium fluoride. The sum of these enthalpies give the standard enthalpy of formation (ΔfH) of lithium fluoride: Δ H f = Δ H sub + IE Li...
29 KB (1,880 words) - 03:13, 4 December 2024