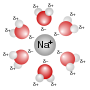
A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [M(H2O)n]z+. The solvation number, n, determined by a...
71 KB (7,653 words) - 21:25, 1 May 2024
hydrogen ions (H+) and hydroxide ions (OH−) are in Arrhenius balance ([H+] [OH−] = Kw = 1 x 10−14 at 298 K). Acids and bases are aqueous solutions, as part...
6 KB (723 words) - 06:50, 21 December 2024
ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have...
15 KB (1,551 words) - 19:45, 27 October 2024
Solvation shell (category Solutions)
around anions and cations from a dissolved salt in a solvent. Metal ions in aqueous solutions form metal aquo complexes. This number can be determined by...
5 KB (642 words) - 09:58, 7 January 2024
derivatives are predicted to be metastable or stable. In aqueous solution, the alkali metal ions form aqua ions of the formula [M(H2O)n]+, where n is the solvation...
214 KB (23,524 words) - 08:55, 14 December 2024
quantitative determination of metal ions. In aqueous solution 8-hydroxyquinoline has a pKa value of ca. 9.9 It reacts with metal ions, losing the proton and...
10 KB (850 words) - 19:13, 22 December 2024
Hydroxide (redirect from Hydroxyl ion)
a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton...
41 KB (4,891 words) - 16:04, 20 November 2024
acid solutions, piranha does not contaminate glassware with Cr3+ ions. Piranha solution is particularly useful when cleaning sintered (or "fritted") glass...
20 KB (2,214 words) - 08:12, 7 November 2024
that are most likely to be present, depending on pH, in aqueous solutions of binary salts of metal ions. The existence must be inferred on the basis of indirect...
41 KB (2,770 words) - 23:49, 2 October 2024
Ethylenediaminetetraacetic acid (category Multiple chemicals in an infobox that need indexing)
confine) metal ions in aqueous solution. In the textile industry, it prevents metal ion impurities from modifying colours of dyed products. In the pulp...
42 KB (4,375 words) - 05:33, 21 October 2024
Hydrochloric acid (redirect from Aqueous Hydrogen Chloride)
chlorine atoms. In aqueous solutions dissociation is complete, with the formation of chloride ions and hydrated hydrogen ions (hydronium ions). A combined...
38 KB (3,995 words) - 21:41, 18 December 2024
von Fehling in 1849. Fehling's solution is prepared by combining two separate solutions: Fehling's A, which is a deep blue aqueous solution of copper(II)...
7 KB (873 words) - 13:31, 4 December 2024
of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is...
10 KB (1,290 words) - 23:34, 15 December 2024
chemical have one ion in common with each other. Hydrogen sulfide (H2S) is a weak electrolyte. It is partially ionized when in aqueous solution, therefore there...
7 KB (931 words) - 20:48, 17 November 2024
Jahn–Teller effect (section Small molecules and ions)
temperature or pressure. Persson, Ingmar (2010). "Hydrated metal ions in aqueous solution: How regular are their structures?". Pure and Applied Chemistry...
61 KB (7,955 words) - 03:19, 5 November 2024
Electrolyte (redirect from Ionic solution)
into ions in solution or in the melt acquires the capacity to conduct electricity. Sodium, potassium, chloride, calcium, magnesium, and phosphate in a liquid...
33 KB (3,443 words) - 00:25, 17 December 2024
Bismuth (category Post-transition metals)
in Inorganic Chemistry and Radiochemistry. Academic Press. pp. 77–78. ISBN 978-0-12-023617-6. Persson, Ingmar (2010). "Hydrated metal ions in aqueous...
61 KB (7,017 words) - 16:06, 5 December 2024
Hydration number (category Solutions)
varies with the atom or ion of interest. In aqueous solution, solutes interact with water molecules to varying degrees. Metal cations form aquo complexes...
10 KB (1,206 words) - 09:32, 4 December 2024
hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen ion concentration...
22 KB (2,336 words) - 16:33, 27 August 2024
HSAB theory (redirect from Soft metal ion)
constants for complexes of many metal ions plus the proton with a wide range of unidentate Lewis acids in aqueous solution, and also offered insights into...
19 KB (2,118 words) - 13:31, 25 October 2024
extraction is possible in non-aqueous systems: In a system consisting of a molten metal in contact with molten salts, metals can be extracted from one...
45 KB (5,649 words) - 21:48, 22 October 2024
purification of aqueous solutions using solid polymeric ion-exchange resin. More precisely, the term encompasses a large variety of processes where ions are exchanged...
20 KB (2,203 words) - 05:28, 21 October 2024
Hydrometallurgy (category All Wikipedia articles written in American English)
metallurgy, the obtaining of metals from their ores. Hydrometallurgy involve the use of aqueous solutions for the recovery of metals from ores, concentrates...
9 KB (1,141 words) - 01:12, 28 October 2023
Lanthanide (redirect from Lanthanide metal)
with nitrogen-donor ligands. The larger ions are 9-coordinate in aqueous solution, [Ln(H2O)9]3+ but the smaller ions are 8-coordinate, [Ln(H2O)8]3+. There...
107 KB (10,324 words) - 12:13, 27 November 2024
voltages involved in these reactions are larger than the potential at which an aqueous solutions would electrolyze. During discharge, lithium ions (Li+ ) carry...
206 KB (21,799 words) - 23:36, 22 December 2024
in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form hydroxide ions OH−...
26 KB (3,041 words) - 06:18, 8 August 2024
monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs"...
31 KB (3,177 words) - 08:15, 23 November 2024
trapping of ions occurs along with the accompanying release of other ions, and thus the process is called ion exchange. There are multiple types of ion-exchange...
24 KB (3,017 words) - 02:10, 15 November 2024
dissociate in aqueous solutions to give OH− (hydroxide ions). In 1923, physical chemists Johannes Nicolaus Brønsted in Denmark and Thomas Martin Lowry in England...
16 KB (1,895 words) - 08:51, 9 December 2024
activity of ions in solution. It is a transducer (or sensor) that converts the change in the concentration of a specific ion dissolved in a solution into an...
16 KB (1,380 words) - 00:03, 1 December 2024