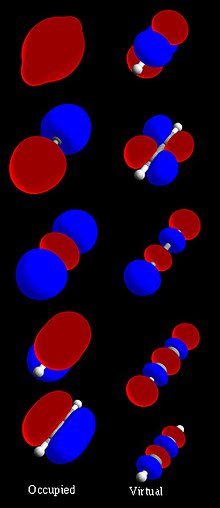
localized orbital sets that include "natural atomic orbitals" (NAO), "natural hybrid orbitals" (NHO), "natural bonding orbitals" (NBO) and "natural (semi-)localized...
5 KB (548 words) - 21:13, 16 October 2022
s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to...
33 KB (3,164 words) - 10:14, 24 April 2024
discussions on localized molecular orbitals, see: natural bond orbital and sigma-pi and equivalent-orbital models.) Molecular orbitals arise from allowed interactions...
35 KB (4,390 words) - 11:31, 6 June 2024
the other two electrons occupy the non-bonding orbital. In the natural bond orbital viewpoint of 3c–4e bonding, the triiodide anion is constructed from...
11 KB (1,215 words) - 21:19, 20 March 2024
Borazine (section Natural bond orbitals (NBO))
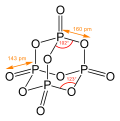
change of formation ΔfH of −531 kJ/mol, is thermally very stable. Natural bond orbital (NBO) analysis suggests weak aromaticity in borazine. In the NBO...
16 KB (1,683 words) - 12:45, 22 June 2024
Wisconsin–Madison. Weinhold is best known for the development of natural bond orbital methods and associated applications in physical and computational...
19 KB (1,956 words) - 08:04, 13 February 2024
molecular orbitals only, and ni is the number of electrons occupying orbital i with coefficients cri and csi on atoms r and s respectively. Assuming a bond order...
9 KB (1,260 words) - 05:21, 12 February 2023
Hydrographic Department Natural hybrid orbitals, one of a set of natural localized orbital sets in quantum chemistry, see Natural bond orbital Takuu language,...
708 bytes (127 words) - 03:47, 9 June 2024
NBO may refer to: National Bank of Oman National Bank Open Natural bond orbital, a model within quantum chemistry Network of Buddhist Organisations Network...
369 bytes (73 words) - 13:22, 8 June 2023
Borole (section Natural bond orbitals)
and 4pz orbitals and the lowest π orbital of the borole ring. Performing natural bond analysis (NBO) on the model pointed to significant orbital interactions...
40 KB (4,514 words) - 18:19, 1 April 2024
computational chemistry, natural resonance theory (NRT) is an iterative, variational functional embedded into the natural bond orbital (NBO) program, commonly...
32 KB (3,001 words) - 20:29, 6 February 2024
Bent's rule (category Chemical bonding)
orbital theory Orbital hybridisation Molecular geometry Linear combination of atomic orbitals Weinhold, F.; Landis, C. L. (2005), Valency and Bonding:...
38 KB (4,243 words) - 07:47, 11 June 2024
sophisticated theories are valence bond theory, which includes orbital hybridization and resonance, and molecular orbital theory which includes the linear...
40 KB (4,876 words) - 20:13, 24 May 2024
OCLC 173809048. Weinhold, Frank; Landis, Clark R. (2005). Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective. Cambridge, UK: Cambridge University...
7 KB (849 words) - 02:55, 17 January 2024
consists of a π orbital, resulting from donation from the planar P lone pair into the empty P orbital of the germylene center. Natural bond orbital analysis...
15 KB (1,761 words) - 16:45, 13 May 2024
Intrinsic bond orbitals (IBO) are localized molecular orbitals giving exact and non-empirical representations of wave functions. They are obtained by unitary...
25 KB (2,927 words) - 04:10, 4 January 2024
The σ-π model and equivalent-orbital model refer to two possible representations of molecules in valence bond theory. The σ-π model differentiates bonds...
8 KB (938 words) - 21:41, 25 October 2023
Conformational isomerism (redirect from Bond rotation barrier)
the Natural Bond Orbital framework. In the staggered conformation, one C-H sigma bonding orbital donates electron density to the antibonding orbital of...
32 KB (3,777 words) - 06:27, 7 February 2024
mesityl ligand. A second-order perturbation theory analysis of the natural bond orbitals (NBOs) of the intermediate in this reaction involved with H2 activation...
26 KB (2,860 words) - 18:33, 31 December 2023
method used to evaluate chalcogen bonding specifically and a wide range of bonding generally is natural bond orbital (NBO) analysis. NBO analysis distinguishes...
16 KB (1,988 words) - 11:12, 10 April 2024
its pi electrons to the empty orbital of Pt, while its π* orbital accepts electrons from Pt. The semilocalized bonding cannot be adequately described...
10 KB (1,195 words) - 02:52, 23 April 2023
Lewis structure (category Chemical bonding)
electron pair repulsion theory Molecular geometry Structural formula Natural bond orbital IUPAC definition of Lewis formula Zumdahl, S. (2005) Chemical Principles...
17 KB (2,359 words) - 07:50, 12 July 2024
Matheus P. (1 January 2013). "The anomeric effect on the basis of natural bond orbital analysis". Organic & Biomolecular Chemistry. 11 (17): 2885–90. doi:10...
19 KB (2,569 words) - 05:10, 15 July 2024
Triboracyclopropenyl (section Structure and bonding)
partitioning theory, the quantum theory of atoms in molecules, natural bond orbital theory, natural orbitals for chemical valence and electron localization function...
27 KB (3,022 words) - 20:12, 25 December 2023
electron density from the carbon–hydrogen σ bonding orbital to the carbon–fluorine σ* antibonding orbital is considered the source of stabilization in...
14 KB (1,566 words) - 07:41, 19 December 2023
FeIII: A Quantum Mechanical Study Based on DFT Computations and Natural Bond Orbital Analyses". Inorg. Chem. 40 (13): 3101–3112. doi:10.1021/ic001258t...
14 KB (1,508 words) - 01:13, 4 June 2023
490 picoseconds. The F-C-C-F dihedral angle is about 72°. Natural bond orbital deletion bond calculations show that 1,2-difluoroethane prefers the gauche...
12 KB (1,105 words) - 03:54, 12 January 2024
interfaced with the valence bond VB2000 and XMVB programs and the Natural Bond Orbital (NBO) population analysis program. The input files use a keyword...
12 KB (1,057 words) - 05:40, 30 April 2024
mechanics/molecular mechanics mixed method using the ONIOM method, natural bond orbital (NBO) analysis and COSMO solvation models. Recently, a highly efficient...
13 KB (1,465 words) - 10:06, 3 December 2023