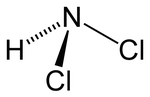
Nitrogen trichloride, also known as trichloramine, is the chemical compound with the formula NCl3. This yellow, oily, and explosive liquid is most commonly...
10 KB (776 words) - 19:24, 30 October 2024
Dichloramine (redirect from Nitrogen dichloride)
chloramines of ammonia, the others being monochloramine (NH2Cl) and nitrogen trichloride (NCl3). This yellow gas is unstable and reacts with many materials...
3 KB (145 words) - 16:47, 13 August 2024
Antimony trichloride is the chemical compound with the formula SbCl3. It is a soft colorless solid with a pungent odor and was known to alchemists as...
10 KB (813 words) - 03:55, 7 January 2024
other actinide trichlorides. Lutetium trichloride, LuCl3 Manganese trichloride, MnCl3 Molybdenum trichloride, MoCl3 Nitrogen trichloride, NCl3, a yellow...
3 KB (300 words) - 21:02, 13 January 2024
Ammonia (redirect from Nitrogen trihydride)
into ammonia, forming nitrogen and hydrogen chloride; if chlorine is present in excess, then the highly explosive nitrogen trichloride (NCl3) is also formed...
139 KB (14,973 words) - 07:42, 11 November 2024
was first found as a product of the thermal decomposition of FN3. Nitrogen trichloride (NCl3) is a dense, volatile, and explosive liquid whose physical...
105 KB (12,228 words) - 19:20, 28 October 2024
other nitrogen trihalides nitrogen trichloride, nitrogen tribromide, and nitrogen triiodide, all of which are explosive. Alone among the nitrogen trihalides...
22 KB (1,964 words) - 06:53, 26 September 2024
Monochloramine (category Nitrogen halides)
compound with the formula NH2Cl. Together with dichloramine (NHCl2) and nitrogen trichloride (NCl3), it is one of the three chloramines of ammonia. It is a colorless...
20 KB (2,045 words) - 01:41, 21 September 2024
Pnictogen (redirect from Nitrogen Group)
acid is unstable. Nitrogen trifluoride is the only stable nitrogen trihalide, with nitrogen trichloride, nitrogen tribromide, and nitrogen triiodide being...
34 KB (4,067 words) - 01:03, 5 November 2024
agene process is a former process for bleaching flour with agene (nitrogen trichloride). The practice was discontinued in 1949 once it became known that...
675 bytes (66 words) - 04:13, 7 July 2022
ammonia. The reaction of bleach with ammonia forms monochloramine, nitrogen trichloride, and a number of other toxic and explosive products depending on...
2 KB (242 words) - 03:26, 7 February 2024
Pool chlorine hypothesis (section Effects of nitrogen trichloride (trichloramine) on the human lung)
known to have been published on the basis of tests of the effects of nitrogen trichloride above chlorinated water on the lung as well as epidemiological evidence...
11 KB (1,352 words) - 18:19, 28 May 2023
was first found as a product of the thermal decomposition of FN3. Nitrogen trichloride (NCl3) is a dense, volatile, and explosive liquid whose physical...
34 KB (4,298 words) - 07:31, 17 May 2024
chlorine, but in reality they react to produce chloramines such as nitrogen trichloride. With excess ammonia and sodium hydroxide, hydrazine may be generated...
55 KB (5,830 words) - 22:52, 13 November 2024
theorise other causes such as poisoning by mercury, mycotoxins, or nitrogen trichloride. During the Vichy government, the supply of grains from field to...
22 KB (2,852 words) - 07:33, 3 October 2024
hydrazine nitrate Nickel hydrazine perchlorate Nitrogen trihalides: Nitrogen trichloride Nitrogen tribromide Nitrogen triiodide Nitroglycerin Nitronium perchlorate...
72 KB (8,212 words) - 08:28, 12 November 2024
Chloramines (category Nitrogen halides)
three compounds: monochloramine (NH2Cl), dichloramine (NHCl2), and nitrogen trichloride (NCl3). Monochloramine is of broad significance as a disinfectant...
5 KB (509 words) - 19:27, 24 October 2024
reduces the final purity of Mg(OH)2. NH4OH, can produce explosive nitrogen trichloride when the brine is used for chlorine production. NaOH as the precipitating...
21 KB (2,031 words) - 11:40, 11 September 2024
favourable. In 1813, when Davy damaged his eyesight in an accident with nitrogen trichloride, he decided to employ Faraday as an assistant. Coincidentally one...
69 KB (7,330 words) - 02:45, 9 November 2024
which in turn produces toxic chlorine gas and can produce explosive nitrogen trichloride. Comet (cleanser) Dichloroisocyanuric acid (Dichlor) Sodium dichloroisocyanurate...
6 KB (444 words) - 15:20, 2 November 2024
monochloramine (NH 2Cl), then dichloramine (NHCl 2), and finally nitrogen trichloride (NCl 3). NH 3 + ClO− → HO− + NH 2Cl NH 2Cl + ClO− → HO− + NHCl 2...
18 KB (1,725 words) - 13:56, 10 October 2024
somewhat arbitrary. Compounds where nitrogen is not assigned −3 oxidation state are not included, such as nitrogen trichloride where the oxidation state is +3;...
13 KB (1,380 words) - 10:14, 3 July 2024
of chlorine. Even though nitrogen in NCl3 is bearing a negative charge, the compound is usually called nitrogen trichloride. Chlorination of metals with...
117 KB (13,065 words) - 23:27, 9 November 2024
mono- is never used with the first element. Thus, NCl3 is termed nitrogen trichloride, BF3 is termed boron trifluoride, and P2O5 is termed diphosphorus...
23 KB (2,775 words) - 07:16, 7 August 2024
NH4N3) III. −RnNXm The halogenated nitrogen group X:halogen (e.g. nitrogen triiodide NI3 and nitrogen trichloride NCl3) IV. −C=N−O− The fulminate group...
3 KB (423 words) - 07:35, 27 January 2023
halogens (fluorine and chlorine) or some of their compounds, such as nitrogen trichloride, are extremely dangerous and can result in explosions. In contrast...
26 KB (2,426 words) - 16:40, 15 May 2024
silver perbromate AgCl silver chloride 7783-90-6 AgCl3Cu2 dicopper silver trichloride 69569-03-5 AgClO3 silver chlorate 7783-92-8 AgClO4 silver perchlorate...
183 KB (107 words) - 22:11, 6 September 2024
the oxides of nitrogen catalysis by metals (1823, with Thénard). Dulong also discovered the dangerously sensitive nitrogen trichloride in 1811, losing...
11 KB (1,244 words) - 01:38, 4 October 2024
theorize other causes such as poisoning by mercury, mycotoxins, or nitrogen trichloride. Pont-Saint-Esprit is twinned with: Egelsbach, Germany Haverhill...
11 KB (822 words) - 03:29, 27 October 2024
Chloroform (redirect from Methyl trichloride)
name "chloroform" is a portmanteau of terchloride (tertiary chloride, a trichloride) and formyle, an obsolete name for the methylylidene radical (CH) derived...
62 KB (5,906 words) - 12:05, 25 October 2024