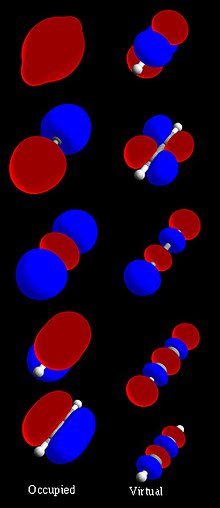
In quantum mechanics, an atomic orbital (/ˈɔːrbɪtəl/) is a function describing the location and wave-like behavior of an electron in an atom. This function...
84 KB (10,978 words) - 06:52, 5 November 2024
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies,...
33 KB (3,170 words) - 15:02, 7 November 2024
which atomic orbital is combined in the term. The coefficients are the weights of the contributions of the n atomic orbitals to the molecular orbital. The...
6 KB (781 words) - 00:03, 6 April 2023
region. The terms atomic orbital and molecular orbital were introduced by Robert S. Mulliken in 1932 to mean one-electron orbital wave functions. At...
35 KB (4,390 words) - 11:31, 6 June 2024
three main requirements for atomic orbital combinations to be suitable as approximate molecular orbitals. The atomic orbital combination must have the correct...
23 KB (3,018 words) - 12:43, 22 October 2024
Electron configuration (redirect from Orbital ordering)
molecule has a different orbital structure. The molecular orbitals are labelled according to their symmetry, rather than the atomic orbital labels used for atoms...
60 KB (6,147 words) - 15:47, 29 October 2024
Azimuthal quantum number (redirect from Orbital quantum number)
quantum number for an atomic orbital that determines its orbital angular momentum and describes aspects of the angular shape of the orbital. The azimuthal quantum...
20 KB (2,143 words) - 19:17, 12 October 2024
orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital...
39 KB (4,968 words) - 12:08, 22 September 2024
unoccupied s-orbital. Because an atom's s-orbital electrons are typically farthest from the nucleus, this results in a significant increase in atomic radius...
29 KB (1,865 words) - 04:36, 14 October 2024
'atomic units', defined as follows: Unit of length, a H = h 2 / 4 π 2 m e 2 {\displaystyle a_{\text{H}}=h^{2}\,/\,4\pi ^{2}me^{2}} , on the orbital...
24 KB (2,367 words) - 14:25, 17 October 2024
Additionally, the cover art on three of their albums showcase stylised atomic orbitals. Orbital have been critically and commercially successful, known particularly...
36 KB (3,623 words) - 01:50, 13 November 2024
original atomic level and one higher. The orbital which is in a lower energy state than the orbitals of the separate atoms is the bonding orbital, which...
7 KB (880 words) - 16:28, 11 November 2024
one-electron wave functions known as spin-orbitals. For an atomic orbital calculation, these are typically the orbitals for a hydrogen-like atom (an atom with...
31 KB (4,730 words) - 17:08, 7 October 2024
which binds electrons in atoms. Some resemblance to atomic orbital models may be seen in a small atomic nucleus like that of helium-4, in which the two protons...
32 KB (3,937 words) - 17:36, 13 November 2024
Atom (redirect from Atomic chemical)
from the original on 7 December 2006. Manthey, David (2001). "Atomic Orbitals". Orbital Central. Archived from the original on 10 January 2008. Herter...
126 KB (12,933 words) - 22:22, 13 November 2024
Bohr model (redirect from Bohr's Atomic Theory)
line frequencies with the orbits of electrons in his atoms. The connection he adopted associated the atomic electron orbital angular momentum with the...
77 KB (10,481 words) - 16:23, 4 November 2024
orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of...
12 KB (1,519 words) - 17:16, 5 November 2024
Core electron (redirect from Core orbital)
valence electrons can be described with atomic orbital theory. In atoms with a single electron the energy of an orbital is determined exclusively by the principal...
10 KB (1,396 words) - 17:09, 11 June 2024
Look up orbital in Wiktionary, the free dictionary. Orbital may refer to: Atomic orbital Molecular orbital Hybrid orbital Orbit Earth orbit Orbit (anatomy)...
1 KB (186 words) - 21:00, 12 November 2024
An electron orbital may refer to: An atomic orbital, describing the behaviour of an electron in an atom A molecular orbital, describing the behaviour...
472 bytes (94 words) - 08:06, 27 October 2017
Slater-type orbitals (STOs) are functions used as atomic orbitals in the linear combination of atomic orbitals molecular orbital method. They are named...
15 KB (2,484 words) - 06:41, 17 November 2023
concerned, not only did this yield a better overall description, i.e. the atomic orbital model, but it also provided a new theoretical basis for chemistry (quantum...
9 KB (1,123 words) - 20:41, 9 September 2024
Sigma bond (redirect from Sigma molecular orbital)
overlapping of atomic orbitals. The concept of sigma bonding is extended to describe bonding interactions involving overlap of a single lobe of one orbital with...
8 KB (918 words) - 13:02, 22 September 2024
Effective nuclear charge (redirect from Orbital penetration effect)
the mean radius of the orbital for hydrogen, and ⟨ r ⟩ Z {\displaystyle \langle r\rangle _{Z}} is the mean radius of the orbital for a proton configuration...
13 KB (1,201 words) - 14:50, 19 October 2024
molecular orbital). According to molecular orbital theory, molecular orbitals are often modeled by the linear combination of atomic orbitals. In a simple...
5 KB (660 words) - 20:31, 24 June 2023
Pi bond (redirect from Pi bonding molecular orbital)
orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has...
7 KB (853 words) - 12:26, 22 September 2024
basis atomic orbitals (AO) and molecular orbitals (MO): Atomic orbital → NAO → NHO → NBO → NLMO → Molecular orbital Natural (localized) orbitals are used...
5 KB (548 words) - 21:13, 16 October 2022
Angular momentum coupling (redirect from Orbit-orbit coupling)
of its atomic nucleus. If we ignore the electron–electron interaction (and other small interactions such as spin–orbit coupling), the orbital angular...
17 KB (2,228 words) - 20:09, 21 February 2024
Magnetic quantum number (redirect from Orbital magnetic quantum number)
of the orbital angular momentum that lies along a given axis, conventionally called the z-axis, so it describes the orientation of the orbital in space...
10 KB (1,196 words) - 18:57, 23 May 2024
Periodic table (redirect from Atomic table)
new electron in a 2p orbital; carbon (1s2 2s2 2p2) fills a second 2p orbital; and with nitrogen (1s2 2s2 2p3) all three 2p orbitals become singly occupied...
250 KB (26,986 words) - 04:50, 11 November 2024