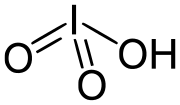
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one...
22 KB (1,761 words) - 14:55, 11 October 2024
refers to an acidic oxide, an oxide that reacts with water to form an oxyacid (an inorganic acid that contains oxygen or carbonic acid), or with a base...
982 bytes (142 words) - 22:40, 26 October 2023
The Nickel oxyacid salts are a class of chemical compounds of nickel with an oxyacid. The compounds include a number of minerals and industrially important...
34 KB (2,428 words) - 09:46, 3 January 2024
peroxonitric acid is a chemical compound with the formula HNO 4. It is an oxyacid of nitrogen, after peroxynitrous acid. Peroxynitrate, the conjugate base...
3 KB (168 words) - 17:29, 29 January 2023
Peroxymonophosphoric acid (H3PO5) is an oxyacid of phosphorus. It is a colorless viscous oil. Its salts are called peroxymonophosphates. Another peroxyphosphoric...
10 KB (933 words) - 10:36, 24 January 2024
of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound H zA xO y. The structures of condensed oxyanions...
18 KB (2,163 words) - 14:15, 13 August 2024
Sulfur oxoacid (redirect from Sulfur oxyacid)
Sulfur oxoacids are chemical compounds that contain sulfur, oxygen, and hydrogen. The best known and most important industrially used is sulfuric acid...
5 KB (137 words) - 14:25, 4 January 2024
phosphorus spontaneously disproportionates to phosphine and various phosphorus oxyacid salts. Many reactions of white phosphorus involve insertion into the P-P...
12 KB (1,162 words) - 10:09, 14 November 2024
Hypophosphorous acid (HPA), or phosphinic acid, is a phosphorus oxyacid and a powerful reducing agent with molecular formula H3PO2. It is a colorless...
14 KB (1,345 words) - 02:53, 22 November 2024
Peroxydiphosphoric acid (H4P2O8) is an oxyacid of phosphorus. Its salts are known as peroxydiphosphates. It is one of two peroxyphosphoric acids, along...
3 KB (317 words) - 03:49, 7 November 2021
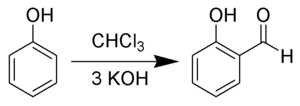
chloroform on phenol and especially on the alkaline solution of aromatic oxyacids]. Berichte der Deutschen Chemischen Gesellschaft (in German). 9: 824–828...
8 KB (796 words) - 09:30, 7 August 2024
Hypofluorous acid, chemical formula HOF, is the only known oxyacid of fluorine and the only known oxoacid in which the main atom gains electrons from...
6 KB (493 words) - 00:21, 13 February 2024
Iodous acid (section Other oxyacids)
disproportionate to molecular iodine and iodates. Iodous acid is part of a series of oxyacids in which iodine can assume oxidation states of −1, +1, +3, +5, or +7. A...
2 KB (90 words) - 18:22, 31 August 2024
ion H2SO3 sulfurous acid SO2 sulfur dioxide Sulfite esters Other sulfur oxyacids Sulfur trioxide (uncharged SO3; a sulfate precursor) Grant v The Australian...
19 KB (1,745 words) - 14:49, 23 November 2024
A halous acid, also known as a halogenous acid, is an oxyacid consisting of a halogen atom in the +3 oxidation state single-bonded to a hydroxyl group...
5 KB (417 words) - 06:35, 25 September 2023
rule, e.g. hydrazoic acid, HN3 Binary acids are often contrasted with oxyacids, which are acids that contain oxygen and other compounds. However, other...
3 KB (317 words) - 20:35, 8 August 2024
the oxidation state of chlorine decreases. The strengths of the chlorine oxyacids increase very quickly as the oxidation state of chlorine increases due...
117 KB (13,065 words) - 23:27, 9 November 2024
Most of the common polyatomic anions are oxyanions, conjugate bases of oxyacids (acids derived from the oxides of non-metallic elements). For example,...
11 KB (773 words) - 20:48, 12 November 2024
polyprotic acids (see Polyprotic acids below), and one to estimate the pKa of oxyacids based on the number of =O and −OH groups (see Factors that affect pKa values...
103 KB (11,513 words) - 22:40, 15 October 2024
Iodic acid (section Other oxyacids)
iodine content of salt.[citation needed] Iodic acid is part of a series of oxyacids in which iodine can assume oxidation states of −1, +1, +3, +5, or +7. A...
8 KB (644 words) - 02:04, 12 September 2024
Periodic acid (section Other oxyacids)
orthoperiodic acid with catalyst PCC. Periodic acid is part of a series of oxyacids in which iodine can assume oxidation states of −1, +1, +3, +5, or +7. A...
12 KB (976 words) - 01:21, 2 November 2024
oxide is accomplished by burning erbium metal, erbium oxalate or other oxyacid salts of erbium. Erbium oxide is insoluble in water and slightly soluble...
34 KB (4,174 words) - 09:09, 25 November 2024
the oxidation number, according to the following scheme: Thus the four oxyacids of chlorine are called hypochlorous acid (HOCl), chlorous acid (HOClO)...
13 KB (1,468 words) - 15:58, 27 September 2024
Ka and a lower pKa than weaker acids. Sulfonic acids, which are organic oxyacids, are a class of strong acids. A common example is toluenesulfonic acid...
47 KB (5,956 words) - 18:26, 8 July 2024
A hypohalous acid is an oxyacid consisting of a hydroxyl group single-bonded to any halogen. Examples include hypofluorous acid, hypochlorous acid, hypobromous...
4 KB (396 words) - 20:46, 10 May 2022
Friesen KJ (2009). "Extending the semi-empirical PM6 method for carbon oxyacid pKa prediction to sulfonic acids: Application towards congener-specific...
54 KB (5,375 words) - 06:11, 4 August 2024
Borinic acid, also known as boronous acid, is an oxyacid of boron with formula H 2BOH. Borinate is the associated anion of borinic acid with formula H...
18 KB (1,880 words) - 20:34, 8 August 2024
Hypoiodous acid (section Other oxyacids)
disproportionate to form iodides and iodates. Hypoiodous acid is part of a series of oxyacids in which iodine can assume oxidation states of −1, +1, +3, +5, or +7. A...
4 KB (195 words) - 14:46, 29 August 2024
trihalides. Antimony forms antimony(III) oxide and antimonite but not oxyacids. Its trihalides, antimony trifluoride, antimony trichloride, antimony tribromide...
34 KB (4,067 words) - 01:03, 5 November 2024
chalcogens, is further reflected in the reported formation of various other oxyacid salts, such as a basic selenate 2TeO2·SeO3 and an analogous perchlorate...
246 KB (27,797 words) - 13:04, 27 August 2024