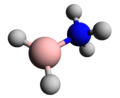
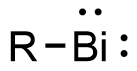Pinacolborane is the borane with the formula (CH3)4C2O2BH. Often pinacolborane is abbreviated HBpin. It features a boron hydride functional group incorporated...
5 KB (446 words) - 11:30, 5 January 2024
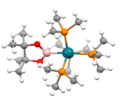
acidic conditions. The B-B bond length is 1.711(6) Å. Dehydrogenation of pinacolborane provides an alternative route: 2 (CH3)4C2O2BH → (CH3)4C2O2B-BO2C2(CH3)4...
4 KB (271 words) - 14:11, 2 August 2024
boron trichloride to produce useful synthetic intermediates such as pinacolborane, bis(pinacolato)diboron, and pinacolchloroborane. Pinacol coupling reaction...
3 KB (169 words) - 17:43, 17 July 2024
stoichiometric equivalent rather than a large excess using the cheaper pinacolborane: Unlike in ordinary electrophilic aromatic substitution (EAS) where...
26 KB (2,605 words) - 12:51, 11 October 2024
of alkenes. Hydroborations have been studied with catecholborane and pinacolborane. It is also active for the hydrosilylation of alkenes. In the presence...
13 KB (1,208 words) - 21:25, 19 August 2024
catalytic hydroboration, pinacolborane and catecholborane are widely used. They also exhibit higher reactivity toward alkynes. Pinacolborane is also widely used...
13 KB (1,481 words) - 05:48, 22 August 2024
aryloxy groups that diminish the Lewis acidity of the boron centre. Pinacolborane adopts a similar structure. Catecholborane is less reactive in hydroborations...
5 KB (352 words) - 15:41, 7 February 2024
Elusive Direct Electrophilic Borylation of Nitrogen Heterocycles with Pinacolborane". Journal of the American Chemical Society. 135 (30): 10978–10981. doi:10...
26 KB (2,860 words) - 20:40, 22 July 2024
compounds, especially those derived from catecholborane and the related pinacolborane, are intermediates in transition metal-catalyzed borylation reactions...
3 KB (305 words) - 08:34, 4 January 2024
2-migratory insertion (M.I.), a transmetalation can take place with pinacolborane (HBPin) to produce the hydroborated product and regenerate ligated copper...
14 KB (1,492 words) - 20:38, 22 July 2024
developed by Mankad (Scheme 5). Bimetallic cleavage of the B-H bond in pinacolborane generates a copper hydride (IPrCu-H) and an iron boryl [(pin)B-Fp],...
15 KB (1,734 words) - 10:40, 26 September 2024
organoboronic acids, and addition and coupling reactions of diborons and pinacolborane for the synthesis of organoboronic esters. Working with Akira Suzuki...
9 KB (1,071 words) - 18:10, 8 February 2023
upon reaction with N2O. In either case, reduction of the product with pinacolborane (HBpin) returns the bismuth(III) centers to the bismuth(I) state and...
39 KB (4,397 words) - 19:03, 12 May 2024