Silver chlorate (AgClO3) forms white, tetragonal crystals. Like all chlorates, it is water-soluble and an oxidizing agent. As a simple metal salt, it...
3 KB (187 words) - 01:41, 17 September 2024
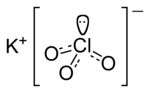
Potassium chlorate is the inorganic compound with the molecular formula KClO3. In its pure form, it is a white solid. After sodium chlorate, it is the...
15 KB (1,457 words) - 01:11, 17 December 2024
should be kept away from silver equipment. Salts of silver with strongly oxidising acids such as silver chlorate and silver nitrate can explode on contact...
95 KB (11,391 words) - 18:07, 2 January 2025
AgNO3 → AgOCl + HNO3 Silver hypochlorite is very unstable, and its solution will soon disproportionate into silver chlorate and silver chloride: 3 AgOCl...
4 KB (263 words) - 16:06, 3 December 2024
detonates the silver fulminate. A fulminate mixture with 10–20% potassium chlorate is cheaper and more brisant than the fulminate alone. Silver fulminate...
9 KB (948 words) - 15:35, 23 January 2024
decomposes at 156 °C to form silver chloride. It can also decompose to silver chlorate is chlorous acid is present. Silver chlorite reacts explosively...
6 KB (358 words) - 01:21, 9 September 2024
– AgBrO3 Silver bromide – AgBr Silver chlorate – AgClO3 Silver chloride – AgCl Silver chromate – Ag2CrO4 Silver fluoroborate – AgBF4 Silver fulminate...
119 KB (8,721 words) - 20:49, 6 December 2024
Silver hypoiodite is a chemical compound with the chemical formula AgIO. This is an ionic compound of silver and the polyatomic ion hypoiodite. Adding...
3 KB (129 words) - 08:00, 28 December 2024
Silver hypobromite is a chemical compound with the chemical formula AgBrO. This is an ionic compound of silver and the polyatomic ion hypobromite. Oxidizing...
3 KB (178 words) - 22:33, 6 December 2024
Sodium hypochlorite (redirect from Sodium chlorate(I))
sodium chlorate and sodium chloride: 3 NaOCl(aq) → 2 NaCl(aq) + NaClO3(aq) This reaction is exploited in the industrial production of sodium chlorate. An...
55 KB (5,824 words) - 16:49, 18 December 2024
Copper(II) chlorate is a chemical compound of the transition metal copper and the chlorate anion with basic formula Cu(ClO3)2. Copper chlorate is an oxidiser...
9 KB (743 words) - 17:41, 17 November 2024
32 0.57 0.94 1.33 Silver bromide AgBr 1.328×10−5 Silver carbonate Ag2CO3 0.003489 Silver chlorate AgClO3 10.4 15.3 20.9 26.8 Silver chloride AgCl 1.923×10−4...
84 KB (196 words) - 10:05, 30 October 2024
AgC2H3O2 silver acetate 563–63–3 AgCl silver chloride 7783–90–6 AgClO3 silver chlorate 7783–92–8 AgClO4 silver perchlorate 7783–93–9 AgF silver fluoride...
139 KB (120 words) - 17:07, 15 July 2024
AgBrO4 silver perbromate AgCl silver chloride 7783-90-6 AgCl3Cu2 dicopper silver trichloride 69569-03-5 AgClO3 silver chlorate 7783-92-8 AgClO4 silver perchlorate...
183 KB (107 words) - 07:29, 24 November 2024
Silver iodate (AgIO3) is a light-sensitive, white crystal composed of silver, iodine and oxygen. Unlike most metal iodates, it is practically insoluble...
3 KB (158 words) - 05:49, 11 September 2024
oxidizer (e.g. potassium chlorate or perchlorate) Ammonal: Ammonium nitrate and aluminium powder Armstrong's mixture: Potassium chlorate and red phosphorus...
68 KB (7,987 words) - 13:12, 20 November 2024
standard processes, but rather than using ferric oxalate plus potassium chlorate as the restrainer (which is ineffective for palladium), a weak solution...
19 KB (2,249 words) - 03:42, 14 October 2024
Silver perchlorate is the chemical compound with the formula AgClO4. This white solid forms a monohydrate and is mildly deliquescent. It is a useful source...
5 KB (299 words) - 15:40, 23 January 2024
Native metal (section Silver)
resembles that of silver, ranging from oxides of its multiple oxidation states through sulfides and silicates to halides and chlorates, iodates, nitrates...
11 KB (1,230 words) - 15:00, 12 July 2024
Hypochlorous acid (redirect from Hydrogen chlorate(I))
hypochlorite Potassium hypochlorite Calcium hypochlorite Barium hypochlorite Silver hypochlorite Methyl hypochlorite tert-Butyl hypochlorite Acetyl hypochlorite...
48 KB (5,441 words) - 13:29, 13 December 2024
20th century. Mercury fulminate has the distinct advantage over potassium chlorate of being non-corrosive, but it is known to weaken with time, by decomposing...
7 KB (568 words) - 17:23, 31 December 2024
Thénard of Paris. The head of the match consisted of a mixture of potassium chlorate, sulfur, gum arabic and sugar. The match was ignited by dipping its tip...
38 KB (4,593 words) - 20:26, 20 December 2024
in the Smith-Gardiner blasting cap by the addition of 10-20% potassium chlorate. This compound was superseded by others: lead azide, lead styphnate, some...
30 KB (3,133 words) - 00:05, 8 November 2024
oxidations with potassium chlorate resulted in a violet emission. Subsequent developments revealed that oxidations with the chlorates of barium, strontium...
83 KB (9,113 words) - 14:54, 3 January 2025
important salt is sodium chlorate, mostly used to make chlorine dioxide to bleach paper pulp. The decomposition of chlorate to chloride and oxygen is...
117 KB (13,073 words) - 15:40, 1 January 2025
V. −OClO2, −OClO3 The chlorate and perchlorate groups respectively, connected to organics/inorganics (e.g. potassium chlorate KClO3, fluorine perchlorate...
3 KB (421 words) - 12:39, 30 December 2024
influence the flame temperature, both by increasing it (e.g. nitrates, chlorates) and decreasing it (e.g. carbonates, oxalates), indirectly influencing...
14 KB (581 words) - 01:21, 26 December 2024
potassium chlorate, producing unstable, gradually decomposing ammonium chlorate; such combination has to be avoided. Nitronium perchlorate Chlorates (also...
28 KB (3,515 words) - 08:14, 4 July 2024
acidity requirement over what had been required, and that also adding a chlorate to the nitrates that were already used would additionally permit running...
16 KB (1,994 words) - 03:17, 24 December 2024
(ClO−, the active ingredient in chlorine bleach), chlorine dioxide (ClO2), chlorate (ClO− 3), and perchlorate (ClO− 4). In terms of its acid–base properties...
18 KB (1,813 words) - 13:56, 10 December 2024