Sodium bifluoride is the inorganic compound with the formula Na[HF2]. It is a salt of sodium cation (Na+) and bifluoride anion ([HF2]−). It is a white...
8 KB (691 words) - 11:12, 13 April 2023
bifluoride anion ([HF2]−). The salt is used as an etchant for glass. Sodium bifluoride is related and is also of commercial use as an etchant as well as...
4 KB (237 words) - 00:29, 25 September 2023
the bifluoride: H2[SiF6] + 6 NH3 + 2 H2O → SiO2 + 6 [NH4]F 2 [NH4]F → NH3 + [NH4][HF2] The resulting ammonium bifluoride is converted to sodium bifluoride...
9 KB (682 words) - 14:49, 24 January 2024
solutions containing HF, sodium fluoride precipitates as the bifluoride salt sodium bifluoride (NaHF2). Heating the latter releases HF and gives NaF. HF...
21 KB (1,996 words) - 05:00, 18 August 2024
Sodium fluorosilicate is a compound with the chemical formula Na2[SiF6]. Unlike other sodium salts, it has a low solubility in water. Sodium hexafluorosilicate...
4 KB (183 words) - 07:08, 22 August 2024
sours: type I is sodium silicofluoride and sodium acid fluoride in powdered, crystal, or flake form; type II is ammonium bifluoride in flake form. Harry...
1 KB (124 words) - 05:41, 27 July 2024
absorb hydrogen fluoride to form a bifluoride salt. Fluorine is produced by the electrolysis of molten potassium bifluoride. This electrolysis proceeds more...
9 KB (725 words) - 18:53, 9 June 2024
gives fluorine. Solutions of inorganic fluorides in water contain F− and bifluoride HF− 2. Few inorganic fluorides are soluble in water without undergoing...
47 KB (5,057 words) - 12:15, 25 August 2024
Difluoride (section Bifluorides)
ion rather than as F− anions. Ammonium bifluoride Potassium bifluoride Sodium bifluoride Ethanedioyl difluoride Ethylidene difluoride Carbonyl difluoride...
6 KB (436 words) - 04:56, 29 May 2024
Sodium hexafluoroantimonate is an inorganic chemical compound with the chemical formula NaSbF6. Sodium hexafluoroantimonate may be synthesised by oxidation...
3 KB (184 words) - 11:21, 16 June 2024
NaHCO3 sodium bicarbonate 144–55–8 NaHF2 sodium bifluoride 1333–83–1 NaHS sodium bisulfide 16721–80–5 NaHSO3 sodium bisulfite 7631–90–5 NaI sodium iodide...
139 KB (120 words) - 17:07, 15 July 2024
Sodium hexafluoroarsenate is an inorganic chemical compound with the chemical formula NaAsF6. Sodium hexafluoroarsenate can be obtained by the fluorination...
3 KB (163 words) - 12:48, 15 June 2024
fluorine, F2, by electrolysis of a solution of HF and potassium bifluoride. The potassium bifluoride is needed because anhydrous HF does not conduct electricity...
15 KB (1,375 words) - 21:30, 23 August 2024
acid. Evaporation of the solution forms crystals of potassium bifluoride. The bifluoride on heating yields potassium fluoride: K 2 CO 3 + 4 HF ⟶ 2 KHF...
7 KB (519 words) - 02:51, 10 April 2024
HCl− 2 ions – the latter, in any case, are much less stable than the bifluoride ions (HF− 2) due to the very weak hydrogen bonding between hydrogen and...
117 KB (13,028 words) - 12:00, 13 August 2024
Sodium hexafluorotitanate is an inorganic compound of sodium, fluorine, and titanium with the chemical formula Na2TiF6. The compound forms white powder...
3 KB (127 words) - 12:55, 14 June 2024
MXenes (section Sodium-ion batteries)
contain a fluoride ion (F−), such as hydrofluoric acid (HF), ammonium bifluoride (NH4HF2), and a mixture of hydrochloric acid (HCl) and lithium fluoride...
74 KB (8,401 words) - 06:23, 8 August 2024
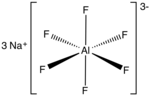
Sodium hexafluoroaluminate is an inorganic compound with formula Na3AlF6. This white solid, discovered in 1799 by Peder Christian Abildgaard (1740–1801)...
7 KB (617 words) - 19:00, 9 June 2024
Sodium hexafluorozirconate (Na2ZrF6) is a chemical compound which can also be referred to as sodium zirconium fluoride. It is an inorganic compound that...
3 KB (130 words) - 10:40, 5 April 2024
(a flux), sodium fluoride (water fluoridation), potassium fluoride (flux), and ammonium fluoride (various). Sodium and potassium bifluorides are significant...
37 KB (3,685 words) - 03:37, 8 August 2024
very short hydrogen bond (114.5 pm) that is similar to the length in the bifluoride ion HF− 2 (114 pm). In aqueous solution the hydroxide ion forms strong...
41 KB (4,891 words) - 13:03, 26 August 2024
Henri Moissan, a chemist in Paris, performed electrolysis on potassium bifluoride dissolved in anhydrous hydrogen fluoride, and successfully isolated fluorine...
52 KB (5,499 words) - 06:28, 7 July 2024
(homoassociation) Overall: 3 HF ⇌ {\displaystyle \rightleftharpoons } HF2− + H2F+ The bifluoride anion (HF2−) encourages the ionization of HF by stabilizing the F−. Thus...
3 KB (290 words) - 23:17, 15 November 2023
HF molecules undergo homoassociation to form polyatomic ions (such as bifluoride, HF− 2) and protons, thus greatly increasing the acidity. This leads to...
21 KB (2,080 words) - 22:11, 15 August 2024
sources include glass-etching or chrome-cleaning agents like ammonium bifluoride or hydrofluoric acid, industrial exposure to fluxes used to promote the...
24 KB (2,501 words) - 13:59, 20 May 2024
NaNH2 Sodium aluminate – NaAlO2 Sodium arsenate – H24Na3AsO16 Sodium azide – NaN3 Sodium bicarbonate – NaHCO3 Sodium biselenide – NaSeH Sodium bisulfate...
119 KB (8,729 words) - 06:43, 23 August 2024
and HI− 2 ions – the latter, in any case, are much less stable than the bifluoride ions (HF− 2) due to the very weak hydrogen bonding between hydrogen and...
106 KB (11,790 words) - 10:37, 13 August 2024
devised a method to produce anhydrous samples from acidified potassium bifluoride; instead, he discovered that the resulting (dry) hydrogen fluoride did...
157 KB (15,346 words) - 02:47, 26 August 2024
→ ZrF4 + 2 H2 Zirconium dioxide reacts at 200 °C with solid ammonium bifluoride to give the heptafluorozirconate salt, which can be converted to the tetrafluoride...
5 KB (453 words) - 18:55, 9 June 2024
dioxotetrafluoride, which in turn is obtained from sodium molybdate: Na2MoO4 + 4 HF → Na2MoF4O2 + 2 H2O Heating sodium dioxotetrafluoride to 400 °C gives monomeric...
4 KB (349 words) - 20:15, 17 January 2024