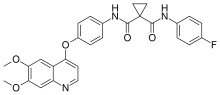
Sorafenib, sold under the brand name Nexavar, is a kinase inhibitor drug approved for the treatment of primary kidney cancer (advanced renal cell carcinoma)...
31 KB (2,651 words) - 05:34, 9 August 2024
partner at Institutional Venture Partners (an original Onyx backer). Sorafenib, co-developed and co-marketed with Bayer and sold under the trade name...
12 KB (1,081 words) - 02:00, 11 November 2024
phase 3 randomized, open-label, clinical trial of Pexa-Vec plus sorafenib versus sorafenib is being conducted on patients with advanced hepatocellular carcinoma...
17 KB (1,775 words) - 12:54, 2 November 2024
months. The most common side effects of Sorafenib include a hand-foot skin reaction and diarrhea. Sorafenib is thought to work by blocking growth of...
91 KB (10,152 words) - 01:40, 14 November 2024
Peginterferon alfa-2b is being studied to treat plexiform neurofibromas. Sorafenib is being studied for treatment of unresectable plexiform neurofibroma...
30 KB (3,257 words) - 16:53, 28 October 2024
people with hepatocellular carcinoma who have been previously treated with sorafenib. The approval was based on CELESTIAL (NCT01908426), a randomized (2:1)...
21 KB (1,640 words) - 06:51, 18 September 2024
and various receptor tyrosine kinases. It is a deuterated derivative of sorafenib with improved pharmacokinetic properties. Keam SJ, Duggan S (November...
5 KB (241 words) - 16:22, 4 July 2024
therapy (IL-2, interferon), kinase inhibitors (temsirolimus, sunitinib, sorafenib, pazopanib) and anti-angiogenic therapies (bevacizumab). "Clear cell carcinoma"...
3 KB (333 words) - 01:07, 3 December 2023
with advanced hepatocellular carcinoma who were previously treated with sorafenib. MetastaticCRC: After the CORRECT trial, two phase 3 trials (CONSIGN,...
11 KB (936 words) - 20:27, 30 August 2024
BRAF (gene) (section Sorafenib)
conformations of the kinase domain as cancer therapeutic candidates. BAY43-9006 (Sorafenib, Nexavar) is a V600E mutant B-Raf and C-Raf inhibitor approved by the...
48 KB (5,285 words) - 16:26, 21 August 2024
molecules that inhibit the tyrosine kinases stimulated by VEGF: sunitinib, sorafenib, axitinib, and pazopanib (some of these therapies target VEGF receptors...
14 KB (1,693 words) - 18:18, 18 October 2024
sorafenib to transition to tivozanib following progression disease but not those on the experimental arm using tivozanib to transition to sorafenib....
15 KB (1,189 words) - 21:51, 11 November 2024
Other therapeutic options in this setting are pazopanib (Votrient), sorafenib (Nexavar), temsirolimus (Torisel), interleukin-2 (Proleukin), everolimus...
36 KB (3,375 words) - 16:01, 9 November 2024
diastolic was reported in the first 24 hours after the first treatment with sorafenib, a VEGF pathway inhibitor.[non-primary source needed] Because these drugs...
33 KB (3,229 words) - 19:54, 7 July 2024
Motesanib Nintedanib Pazopanib Radotinib Quizartinib Ripretinib Sunitinib Sorafenib Toceranib Antibodies: Olaratumab Ramucirumab Tovetumab RET (GFL) SCF (c-Kit)...
72 KB (6,433 words) - 04:45, 17 November 2024
showed significantly extended progression-free survival when compared to sorafenib. In December 2011, the Oncologic Drugs Advisory Committee (ODAC) voted...
16 KB (1,194 words) - 23:36, 16 August 2024
inhibited by sorafenib. Giving sorafenib in combination with rifampicin or inducers of CYP3A4 can decrease plasma concentration of sorafenib. CYP3A4 inhibitors...
51 KB (4,735 words) - 08:15, 2 January 2024
metabolic changes associated with depression in metastatic breast cancer, sorafenib and thyroid cancer, pemetrexed-based chemotherapy in lung cancer, atypical...
31 KB (2,814 words) - 20:17, 25 October 2024
treatment, with the most common toxicity being diarrhea. Imatinib, sunitinib, sorafenib, and pazopanib have been studied in the treatment of aggressive fibromatosis...
10 KB (1,179 words) - 18:31, 8 August 2024
of liver cancer produced sorafenib, a targeted therapy drug that prevents cell proliferation and blood cell growth. Sorafenib obtained FDA approval for...
57 KB (6,080 words) - 09:55, 17 November 2024
Midostaurin Nintedanib Odronextamab Pazopanib Pexidartinib Quizartinib Regorafenib Ripretinib Sorafenib Sunitinib Tebentafusp Tepotinib Vandetanib Venetoclax...
12 KB (821 words) - 02:57, 12 October 2024
Motesanib Nintedanib Pazopanib Radotinib Quizartinib Ripretinib Sunitinib Sorafenib Toceranib Antibodies: Olaratumab Ramucirumab Tovetumab RET (GFL) SCF (c-Kit)...
121 KB (13,799 words) - 10:33, 13 November 2024
Motesanib Nintedanib Pazopanib Radotinib Quizartinib Ripretinib Sunitinib Sorafenib Toceranib Antibodies: Olaratumab Ramucirumab Tovetumab RET (GFL) SCF (c-Kit)...
93 KB (8,729 words) - 06:34, 10 November 2024
Assay, which is used to detect the FLT3 mutation in patients with AML. Sorafenib has been reported to show significant activity against Flt3-ITD positive...
15 KB (1,727 words) - 02:27, 15 October 2024
(OSM) Thymic stromal lymphopoietin (TSLP) Additional cytokine receptor modulators: Emfilermin Lestaurtinib Midostaurin Quizartinib Sorafenib Sunitinib...
26 KB (2,676 words) - 02:12, 19 November 2024
Because of this finding, erlotinib has replaced gefitinib in this setting. Sorafenib (Nexavar) Sunitinib (Sutent) Dasatinib (Sprycel) Lapatinib (Tykerb) Nilotinib...
20 KB (2,234 words) - 01:21, 22 July 2024
followed by bevacizumab 15 mg/kg IV on the same day, every 3 weeks, or sorafenib orally twice daily. In July 2020, it was approved in the United States...
43 KB (4,183 words) - 04:17, 1 October 2024
for clinical use, kinase inhibitors that also inhibit Raf kinases (e.g. Sorafenib) are successful antineoplastic agents against various types of cancer...
46 KB (5,492 words) - 05:56, 16 October 2024
Targeted cancer therapies, especially the tyrosine kinase inhibitors sorafenib and sunitinib, have also been associated with a high incidence of acral...
16 KB (1,638 words) - 17:34, 11 November 2024
N, Furuichi Y, Imai Y (2013). "Hepatocellular carcinoma treated with sorafenib: Early detection of treatment response and major adverse events by contrast-enhanced...
120 KB (13,852 words) - 19:30, 20 November 2024