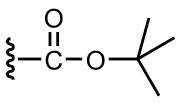
The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group (BOC group) is an acid-labile protecting group used in organic synthesis...
10 KB (1,045 words) - 17:06, 19 July 2024
p-Methoxybenzyloxycarbonyl (Moz or MeOZ) group – Removed by hydrogenolysis, more labile than Cbz tert-Butyloxycarbonyl (Boc) group — Removed by concentrated strong...
57 KB (6,755 words) - 23:19, 9 April 2024
product as its tert-Butyloxycarbonyl protecting group derivative (7). Catalytic hydrogenation then removes the carbobenzyloxy protecting group to afford the...
12 KB (1,169 words) - 08:31, 14 August 2024
eliminated after the protonation of the amino group, and finally the deprotonation of a hydroxyl group produces an amino acid. One example of the Strecker...
10 KB (1,069 words) - 19:12, 26 September 2024
different alkyl sidechains attached. Its synthesis uses a tert-butyloxycarbonyl protecting group (Boc group) to mask the reactivity of one of its nitrogen atoms...
17 KB (1,681 words) - 02:07, 27 September 2024
Peptide synthesis (section Protecting groups schemes)
synthesis relied on tert-butyloxycarbonyl (abbreviated 'Boc') as a temporary N-terminal α-amino protecting group. The Boc group is removed with acid...
54 KB (6,100 words) - 14:21, 6 October 2024
peptide synthesis, the N-terminus of the growing peptide is protected with tert-butyloxycarbonyl while its C-terminus (Z–NH–CH(R)–COOH) is coupled to N-hydroxyphthalimide...
19 KB (1,927 words) - 16:55, 7 March 2024