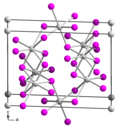
Tin(IV) iodide, also known as stannic iodide, is the chemical compound with the formula SnI4. This tetrahedral molecule crystallizes as a bright orange...
5 KB (274 words) - 22:59, 17 February 2024
Tin iodide may refer to two different ionic compounds. Tin(II) iodide or stannous iodide Tin(IV) iodide or stannic iodide This set index article lists...
453 bytes (55 words) - 00:20, 18 February 2022
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride, is an inorganic compound of tin and chlorine with the formula SnCl4. It is a colorless...
9 KB (676 words) - 10:54, 14 July 2024
Tin(IV) acetate is the acetate salt of tin(IV), with the chemical formula of Sn(CH3COO)4. Tin(IV) acetate can be refluxed by thallium acetate and tin(IV)...
5 KB (322 words) - 04:45, 3 August 2023
Tin(II) iodide, also known as stannous iodide, is an ionic tin salt of iodine with the formula SnI2. It has a formula weight of 372.519 g/mol. It is a...
2 KB (102 words) - 02:11, 23 June 2022
Unlike the other tin tetrahalides, tin(IV) chloride, tin(IV) bromide, and tin(IV) iodide, which contain tetrahedrally coordinated tin, tin(IV) fluoride contains...
4 KB (278 words) - 02:49, 3 January 2024
Kristallstrukturen von Zinn(IV)-bromid und -iodid" [Tin halogen compounds. II. The Molecular and Crystal Structures of Tin(IV) Bromide and Tin(IV) Iodide]. Zeitschrift...
5 KB (353 words) - 20:14, 3 September 2024
Tin(II) sulfate – SnSO4 Tin(II) sulfide – SnS Tin(IV) bromide – SnBr4 Tin(IV) chloride – SnCl4 Tin(IV) fluoride – SnF4 Tin(IV) iodide – SnI4 Tin(IV)...
119 KB (8,735 words) - 18:51, 11 November 2024
Tin(IV) sulfide is a compound with the formula Sn S 2. The compound crystallizes in the cadmium iodide motif, with the Sn(IV) situated in "octahedral holes'...
8 KB (658 words) - 15:50, 2 November 2024
57% hydriodic acid: GeO2 + 4 HI → GeI4 + 2 H2O Germanium(IV) iodide reacts with tetraalkyl tin at 250 °C to form R2SnI2 and R2GeI2 (R= Et, Bu, Ph). It...
5 KB (489 words) - 08:15, 26 October 2023
and in electrolytic baths for tin-plating. Tin(II) chloride should not be confused with the other chloride of tin; tin(IV) chloride or stannic chloride...
17 KB (1,714 words) - 16:40, 29 October 2024
tin(IV) sulfide SnS2, crystallises in the cadmium iodide motif, which indicates that Pb should be assigned the formal oxidation state of 4+. Lead(IV)...
2 KB (129 words) - 20:25, 17 January 2024
Silicon tetraiodide (redirect from Silicon(IV) iodide)
tetrabromide Other cations Carbon tetraiodide Germanium tetraiodide Tin(IV) iodide Except where otherwise noted, data are given for materials in their...
5 KB (333 words) - 14:04, 14 May 2024
compounds, only the iodides are colored. Tin(II) chloride (also known as stannous chloride) is the most important commercial tin halide. Illustrating...
80 KB (8,838 words) - 09:36, 25 November 2024
Organotin chemistry (redirect from Organo-tin)
compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. The tetraorgano derivatives...
24 KB (2,623 words) - 18:21, 19 September 2024
SnCl4 tin(IV) chloride 7646–78–8 SnF2 tin(II) fluoride 7783–47–3 SnF4 tin(IV) fluoride 7783–62–2 SnH4 tin(IV) hydride 2406–52–2 SnI2 tin(II) iodide 10294–70–9...
139 KB (120 words) - 17:07, 15 July 2024
Sn(CrO4)2 tin(IV) chromate 10101-75-4 SnI4 tin(IV) iodide 7790-47-8 Sn(OH)2 tin(II) hydroxide Sn(OH)4 tin(IV) hydroxide SnO tin(II) oxide SnO2 tin(IV) oxide...
183 KB (107 words) - 07:29, 24 November 2024
Carbon tetraiodide (redirect from Carbon iodide)
triphenylphosphine (PPh3) and carbon tetraiodide. Alcohols are converted in and to iodide, by a mechanism similar to the Appel reaction. In an Appel reaction, carbon...
6 KB (444 words) - 02:29, 23 October 2024
Seok, Sang Il (30 March 2016). "Fabrication of Efficient Formamidinium Tin Iodide Perovskite Solar Cells through SnF 2 –Pyrazine Complex". Journal of the...
14 KB (1,562 words) - 04:03, 29 July 2024
Titanium tetraiodide (redirect from Titanium(IV) iodide)
titanium. TiI4 is a rare molecular binary metal iodide, consisting of isolated molecules of tetrahedral Ti(IV) centers. The Ti-I distances are 261 pm. Reflecting...
6 KB (478 words) - 03:00, 19 February 2024
Tetramethyltin (redirect from Tin tetramethyl)
of the Grignard reagent methylmagnesium iodide, with tin tetrachloride, which is synthesized by reacting tin metal with chlorine gas. 4 CH3MgI + SnCl4...
6 KB (436 words) - 20:06, 12 June 2023
Iodine (section Hydrogen iodide)
iodide. For example, molybdenum(IV) oxide reacts with aluminium(III) iodide at 230 °C to give molybdenum(II) iodide. An example involving halogen exchange...
104 KB (11,657 words) - 19:32, 24 November 2024
Germanium(II) iodide is an iodide of germanium, with the chemical formula of GeI2. Germanium(II) iodide can be produced by reacting germanium(IV) iodide with hydriodic...
4 KB (346 words) - 04:23, 20 September 2023
tin and copper(II) sulfate: Sn (s) + CuSO4 (aq) → Cu (s) + SnSO4 (aq) Tin(II) sulfate is a convenient source of tin(II) ions uncontaminated by tin(IV)...
4 KB (239 words) - 18:29, 10 April 2024
Tin(II) fluoride, commonly referred to commercially as stannous fluoride (from Latin stannum, 'tin'), is a chemical compound with the formula SnF2. It...
22 KB (2,123 words) - 03:58, 9 November 2024
List of semiconductor materials (redirect from IV semiconductor)
Sapna (2020). "Atomic structure and defect dynamics of monolayer lead iodide nanodisks with epitaxial alignment on graphene". Nature Communications....
54 KB (2,525 words) - 05:44, 8 October 2024
Titanium tetrachloride (redirect from Titanium(IV) chloride)
including vanadyl chloride (VOCl3), silicon tetrachloride (SiCl4), and tin tetrachloride (SnCl4), which must be separated. The world's supply of titanium...
19 KB (1,732 words) - 00:16, 20 June 2024
Uranium (redirect from Uranium(V) iodide)
chlorine. All uranium chlorides react with water and air. Bromides and iodides of uranium are formed by direct reaction of, respectively, bromine and...
110 KB (12,380 words) - 11:06, 17 October 2024
metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste...
52 KB (5,787 words) - 10:39, 15 November 2024
readily into lead(II) chloride and chlorine gas. The bromide and iodide of lead(IV) are not known to exist. Lead dioxide dissolves in alkali hydroxide...
13 KB (1,466 words) - 18:10, 8 August 2024