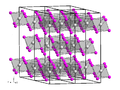
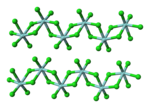
Vanadium tetrachloride is the inorganic compound with the formula VCl4. This reddish-brown liquid serves as a useful reagent for the preparation of other...
5 KB (316 words) - 07:50, 7 February 2023
Titanium tetrachloride, TiCl4 Tungsten tetrachloride, WCl4 Uranium tetrachloride, UCl4, a dark green compound of uranium Vanadium tetrachloride, VCl4, a...
2 KB (195 words) - 20:52, 13 January 2024
management Vanadium carbide – Extremely hard refractory ceramic material Vanadium redox battery – Type of rechargeable flow battery Vanadium tetrachloride – Chemical...
75 KB (8,503 words) - 18:56, 4 August 2024
appear red or orange owing to an impurity of vanadium tetrachloride. VOCl3 is a vanadium compound with vanadium in the +5 oxidation state and as such is diamagnetic...
8 KB (646 words) - 20:00, 30 September 2023
yellow-brown solid that is very hygroscopic. Unlike the corresponding vanadium tetrachloride, the tetrafluoride is not volatile because it adopts a polymeric...
5 KB (242 words) - 16:23, 21 December 2023
complex. Vanadium(V) chloride chlorimide can be made by chlorinating vanadium nitride at 120°. Or chlorine azide can react with vanadium tetrachloride to yield...
6 KB (602 words) - 09:06, 3 January 2024
polymeric with octahedral vanadium(III) surrounded by six bromide ligands. VBr3 has been prepared by treatment of vanadium tetrachloride with hydrogen bromide:...
5 KB (415 words) - 10:58, 30 March 2024
V2O5 Vanadium carbide – VC Vanadium oxytrichloride (Vanadium(V) oxide trichloride) – VOCl3 Vanadium pentafluoride – VF5 Vanadium tetrachloride – VCl4...
119 KB (8,735 words) - 17:31, 25 October 2024
representative example is the reaction of phenol with a solution of vanadium tetrachloride, which yields about 60% yield of three isomeric dihydroxybiphenyl...
12 KB (1,270 words) - 01:20, 30 August 2024
chlorides as well, but most are less volatile than TiCl4. Vanadium tetrachloride and vanadium oxytrichloride codistill with TiCl4, but these impurities...
4 KB (530 words) - 14:34, 16 February 2024
the manufacture of white pigments. Other compounds include titanium tetrachloride (TiCl4), a component of smoke screens and catalysts; and titanium trichloride...
80 KB (8,810 words) - 19:23, 8 October 2024
molybdenum(V) chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium tetrachloride. d2 Examples: titanocene dicarbonyl. d3 Examples: Reinecke's salt...
14 KB (1,701 words) - 03:27, 4 October 2023
University Thesis Part 1: Electron diffraction investigations of vanadium tetrachloride, dimethylketene dimer, tetrachloroethylene, and trichloroethylene...
62 KB (5,965 words) - 13:08, 8 September 2024
can be produced by several related procedures: The reaction of carbon tetrachloride and hafnium oxide at above 450 °C; HfO2 + 2 CCl4 → HfCl4 + 2 COCl2 Chlorination...
13 KB (1,306 words) - 00:57, 23 September 2023
Group 5 element (redirect from Vanadium family)
Group 5 is a group of elements in the periodic table. Group 5 contains vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db). This group lies in the...
80 KB (8,565 words) - 09:59, 26 October 2024
Vanadyl nitrate (redirect from Vanadium oxynitrate)
Vanadyl nitrate, also called vanadium oxytrinitrate or vanadium oxynitrate is an inorganic compound of vanadium in the +5 oxidation state with nitrate...
11 KB (964 words) - 17:01, 27 April 2024
pyrophoric UN 2442 8 Trichloroacetyl chloride UN 2443 8 Vanadium oxytrichloride UN 2444 8 Vanadium tetrachloride UN 2445 4.2 (UN No. no longer in use) Lithium alkyls...
7 KB (103 words) - 17:09, 14 March 2022
complexes to be reported, is prepared from sodium cyclopentadienyl and vanadium tetrachloride: 2 NaC5H5 + VCl4 → VCp2Cl2 + 2NaCl Reduction of this compound gives...
6 KB (678 words) - 17:36, 27 April 2024
Niobium(IV) chloride (redirect from Niobium tetrachloride)
Niobium(IV) chloride, also known as niobium tetrachloride, is the chemical compound of formula NbCl4. This compound exists as dark violet crystals, is...
5 KB (479 words) - 09:55, 20 June 2022
with concentrated sulfuric acid and anhydrous tin tetrachloride: Reaction between tin tetrachloride and sulfuric acid in a 1:2 molar mixture at near reflux...
18 KB (1,557 words) - 20:11, 18 October 2024
(KSCN). The common carbon halides are carbon tetrafluoride (CF4), carbon tetrachloride (CCl4), carbon tetrabromide (CBr4), carbon tetraiodide (CI4), and a...
13 KB (995 words) - 12:40, 1 July 2024
monomeric and thus at least somewhat volatile. It is prepared from titanium tetrachloride (which is also tetrahedral, diamagnetic, and volatile) by treatment...
4 KB (246 words) - 23:56, 26 June 2024
(2004). "New Details Concerning the Reactions of Nitric Oxide with Vanadium Tetrachloride". Inorganic Chemistry. 43 (22): 7227–7233. doi:10.1021/ic0491534...
61 KB (3,477 words) - 14:14, 21 May 2024
discovery. The same year, Berzelius became the first to prepare silicon tetrachloride; silicon tetrafluoride had already been prepared long before in 1771...
86 KB (10,719 words) - 18:00, 18 October 2024
laboratory-scale fluorinating agent. The only stable chlorides are selenium tetrachloride (SeCl4) and selenium monochloride (Se2Cl2), which might be better known...
96 KB (11,087 words) - 08:09, 31 October 2024
member of group 5 in the 6d series of transition metals, placing it under vanadium, niobium, and tantalum. Dubnium should share most properties, such as its...
50 KB (9,127 words) - 00:47, 21 October 2024
It becomes superconductive at temperatures below 1.4 K. Protactinium tetrachloride is paramagnetic at room temperature, but becomes ferromagnetic when...
50 KB (5,847 words) - 19:06, 29 October 2024
135 °C the metal will ignite in air and will explode if placed in carbon tetrachloride. Plutonium is a reactive metal. In moist air or moist argon, the metal...
139 KB (14,972 words) - 03:21, 5 October 2024
also determined an atomic weight of 72.32 by analyzing pure germanium tetrachloride (GeCl 4), while Lecoq de Boisbaudran deduced 72.3 by a comparison of...
71 KB (7,384 words) - 05:25, 26 October 2024
Ore genesis (section Vanadium)
biological process might have played a role in the formation of vanadium ores. Vanadium is also present in fossil fuel deposits such as crude oil, coal...
33 KB (3,971 words) - 04:39, 23 October 2024