In quantum chemistry, the quantum theory of atoms in molecules (QTAIM), sometimes referred to as atoms in molecules (AIM), is a model of molecular and...
12 KB (1,524 words) - 03:50, 9 February 2024
polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may...
33 KB (3,697 words) - 12:06, 12 March 2024
particles such as muons (muonic atoms) or pions (pionic atoms). Because these substitute particles are usually unstable, exotic atoms typically have very short...
12 KB (1,471 words) - 10:43, 3 July 2024
Diatomic molecules (from Greek di- 'two') are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists...
21 KB (2,550 words) - 08:56, 19 June 2024
Chemical bond (section Bonds in chemical formulas)
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force...
40 KB (4,876 words) - 20:13, 24 May 2024
a list of molecules that have been detected in the interstellar medium and circumstellar envelopes, grouped by the number of component atoms. The chemical...
141 KB (9,341 words) - 07:42, 18 July 2024
Ionization (section Ionization energy of atoms)
electron after collisions with subatomic particles, collisions with other atoms, molecules, electrons, positrons, protons, antiprotons and ions, or through the...
48 KB (6,714 words) - 23:23, 5 July 2024
size of atoms and molecules that possess any electrons at all. Thus, anions (negatively charged ions) are larger than the parent molecule or atom, as the...
30 KB (3,020 words) - 20:58, 7 May 2024
Quantum chemistry (redirect from Atom/Wave model)
on the electronic ground state and excited states of individual atoms and molecules as well as the study of reaction pathways and transition states that...
20 KB (2,232 words) - 15:20, 19 June 2024
VSEPR theory (redirect from Molecule shape)
pairs around central atoms in molecules, especially simple and symmetric molecules. A central atom is defined in this theory as an atom which is bonded to...
45 KB (4,038 words) - 13:09, 14 April 2024
History of atomic theory (redirect from Atom theory)
hydrogen atoms. In reality, an ethylene molecule has two carbon atoms and four hydrogen atoms (C2H4), and a methane molecule has one carbon atom and four...
70 KB (9,130 words) - 18:32, 17 July 2024
of an atom or molecule. Irving Langmuir was the first to propose in his 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which,...
60 KB (6,147 words) - 22:00, 18 May 2024
bond theory. In the molecule H 2, the hydrogen atoms share the two electrons via covalent bonding. Covalency is greatest between atoms of similar electronegativities...
28 KB (3,654 words) - 07:13, 1 April 2024
Chemical formula (section Trapped atoms)
be written C2H6O because the molecules of ethanol all contain two carbon atoms, six hydrogen atoms, and one oxygen atom. Some types of ionic compounds...
24 KB (3,205 words) - 14:13, 13 July 2024
Matter (section Based on atoms)
definition because they are made of atoms. This definition can be extended to include charged atoms and molecules, so as to include plasmas (gases of...
81 KB (9,592 words) - 14:33, 22 July 2024
Chemical polarity (redirect from Polar molecules)
charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar...
24 KB (2,751 words) - 13:39, 24 June 2024
Metastability (redirect from Metastability in molecules)
subatomic particles (in atomic nuclei or in atoms) or in molecules, macromolecules or clusters of atoms and molecules. Later, it was borrowed for the study...
16 KB (1,986 words) - 17:48, 30 May 2024
Molecular geometry (redirect from Geometry of molecules)
two atoms bonded together in any given molecule. A bond angle is the angle formed between three atoms across at least two bonds. For four atoms bonded...
23 KB (2,310 words) - 14:26, 4 May 2024
Bohr model (redirect from Atom/Bohr model)
Planck constant could be taken as determining the size of atoms, or that the size of atoms could be taken to determine the Planck constant. Lorentz included...
64 KB (9,061 words) - 00:22, 15 July 2024
Multiplicity (chemistry) (section Atoms)
invalid for this excited state. In organic chemistry, carbenes are molecules which have carbon atoms with only six electrons in their valence shells and therefore...
7 KB (814 words) - 22:54, 5 September 2023
Spectroscopy (category Concepts in astronomy)
temperature of a Black Body. Spectroscopy is used in physical and analytical chemistry because atoms and molecules have unique spectra. As a result, these spectra...
42 KB (4,648 words) - 07:22, 31 May 2024
Thermodynamic temperature (redirect from Atoms can have zero kinetic velocity and simultaneously be vibrating due to zero-point energy)
specific heat capacity per atom and why that value is lowest of all the gases. Molecules (two or more chemically bound atoms), however, have internal structure...
105 KB (13,804 words) - 05:44, 20 July 2024
(hydrogen) atoms in different H2 molecules equals 0.06 kJ/mol (0.6 meV) and the pairwise attractive interaction energy between O (oxygen) atoms in different...
35 KB (3,934 words) - 02:02, 14 July 2024
Ionization energy (section Atoms: values and trends)
of atoms or molecules, usually as kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Comparison of ionization energies of atoms in the...
51 KB (5,883 words) - 15:10, 14 May 2024
Spin quantum number (category All Wikipedia articles written in American English)
spin quantum number S. This occurs especially in light atoms (or in molecules formed only of light atoms) when spin–orbit coupling is weak compared to...
22 KB (2,959 words) - 08:55, 31 May 2024
Molecular dynamics (category All Wikipedia articles written in American English)
simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving...
79 KB (9,900 words) - 19:37, 11 July 2024
History of molecular theory (redirect from History of the molecule concept)
individual atoms of different chemical elements such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules. The modern...
36 KB (4,474 words) - 14:38, 25 April 2024
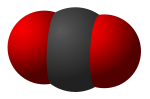
Triatomic molecules are molecules composed of three atoms, of either the same or different chemical elements. Examples include H2O, CO2 (pictured), HCN...
3 KB (378 words) - 01:05, 3 February 2024
Matter wave (section Atoms)
experimentally demonstrated, first for electrons in 1927 and for other elementary particles, neutral atoms and molecules in the years since. At the end of the 19th...
66 KB (7,562 words) - 20:21, 6 June 2024
Energy level (redirect from Quantized energy levels of atoms)
term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can...
22 KB (2,831 words) - 03:08, 12 February 2024