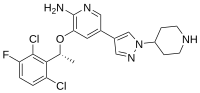
Crizotinib, sold under the brand name Xalkori among others, is an anti-cancer medication used for the treatment of non-small cell lung carcinoma (NSCLC)...
22 KB (1,866 words) - 05:42, 2 November 2024
implications on the response of the tumour and prognosis of the patient. Crizotinib (also a ROS1 and c-MET inhibitor) was approved in Aug 2011 by the US FDA...
17 KB (1,951 words) - 10:39, 25 January 2024
positive non-small cell lung cancer. Treatment with crizotinib achieves 60% response rate. However, crizotinib showed no improvement on overall survival compared...
15 KB (1,597 words) - 20:20, 8 July 2024
metastatic non-small cell lung cancer whose disease has progressed on crizotinib and at least one other ALK inhibitor for metastatic disease or whose disease...
19 KB (1,437 words) - 21:45, 6 November 2024
include velpatasvir/sofosbuvir, sofosbuvir/daclatasvir, osimertinib, crizotinib, daclatasvir, sofosbuvir, afatinib, axitinib, brigatinib, baricitinib...
5 KB (232 words) - 10:23, 8 March 2024
treatment of adults with ALK‑positive advanced NSCLC previously treated with crizotinib. In the United States, it is indicated for the treatment of people with...
23 KB (1,837 words) - 19:36, 11 November 2024
been increasing. In 2016, the small molecule tyrosine kinase inhibitor, crizotinib, was approved for the treatment of patients with metastatic NSCLC whose...
29 KB (3,682 words) - 16:09, 27 October 2024
responses to crizotinib while 1 patient previously treated with a corticosteroid, prednisone, continued to have progressive disease on crizotinib; and 3) 6...
33 KB (3,914 words) - 20:05, 30 August 2023
patients. Crizotinib, which gained FDA approval in August 2011, is an inhibitor of several kinases, specifically ALK, ROS1, and MET. Crizotinib has been...
51 KB (5,336 words) - 08:52, 11 November 2024
inhibitors such as crizotinib showed to be effective against tumors harboring ALK fusions. Most patients previously treated with crizotinib benefited from...
42 KB (4,606 words) - 14:59, 6 November 2024
in hyperactive ALK protein, which can be treated with ALK inhibitors crizotinib, or its successors alectinib, brigatinib, and ceritinib. Those treated...
90 KB (9,784 words) - 07:53, 10 October 2024
Previously, it was only indicated for patients who had developed resistant to crizotinib, another ALK inhibitor, but has since had its usage expanded to serve...
16 KB (1,264 words) - 12:39, 14 June 2024
Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a MK-2461 PF-04217903...
93 KB (8,729 words) - 06:34, 10 November 2024
affected by ALK+ lymphoma with an ALK inhibitor (crizotinib). The events which led to the discovery of crizotinib for the treatment of ALK+ lymphomas became...
6 KB (634 words) - 17:22, 22 August 2024
kinase inhibitors, e.g. FDA approved tofacitinib ALK inhibitors, e.g. crizotinib Bcl-2 inhibitors (e.g. FDA approved venetoclax, obatoclax in clinical...
20 KB (2,234 words) - 01:21, 22 July 2024
Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a MK-2461 PF-04217903...
121 KB (13,799 words) - 18:53, 22 October 2024
(Toceranib), was also approved for cancer in dogs, and the ALK inhibitor Crizotinib also grew out of a SUGEN program. In October 2006, the company announced...
168 KB (14,575 words) - 07:54, 11 November 2024
Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a MK-2461 PF-04217903...
69 KB (6,096 words) - 08:19, 11 November 2024
currently[when?] in clinical trials. Crizotinib and cabozantinib were the first to be approved by the U.S. FDA. Crizotinib received accelerated approval in...
32 KB (3,542 words) - 19:34, 2 July 2024
Aumolertinib L01EC01 Vemurafenib L01EC02 Dabrafenib L01EC03 Encorafenib L01ED01 Crizotinib L01ED02 Ceritinib L01ED03 Alectinib L01ED04 Brigatinib L01ED05 Lorlatinib...
12 KB (877 words) - 15:36, 25 January 2024
Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a MK-2461 PF-04217903...
34 KB (3,021 words) - 12:39, 5 August 2024
Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a MK-2461 PF-04217903...
42 KB (3,935 words) - 20:28, 27 October 2024
crisaborole crisnatol (INN) Criticare HN Crixivan Crizanlizumab-tmca crizotinib (USAN), INN) croconazole (INN) CroFab Crofelemer crolibulin (USAN, INN)...
7 KB (417 words) - 00:11, 9 August 2024
monotherapy and in combination with other agents, such as binimetinib and crizotinib. Cao L, Chen S, Sun R, Ashby CR, Wei L, Huang Z, Chen ZS (2023). "Darovasertib...
5 KB (183 words) - 18:53, 11 November 2024
Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a MK-2461 PF-04217903...
5 KB (412 words) - 23:17, 19 June 2023
started at Sugen also contributed to the development of the ALK inhibitor crizotinib (Xalkori), FDA-approved for NSCLC in 2011. Sugen also generated extensive...
8 KB (823 words) - 17:00, 26 September 2024
Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a MK-2461 PF-04217903...
13 KB (1,177 words) - 01:44, 4 November 2024
Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a MK-2461 PF-04217903...
149 KB (15,703 words) - 16:01, 5 November 2024
worsened after use or were unable to take another medication, Xalkori (crizotinib). Immunotherapy, for instance Anti-PD-1 alone or in combination with anti-CTLA-4...
31 KB (3,121 words) - 02:48, 30 September 2024
Vandetanib (VEGFR, EGFR). c-MET inhibitors: Cabozantinib (VEGFR), Capmatinib, Crizotinib (ALK) Non-receptor bcr-abl Asciminib Bosutinib Dasatinib Imatinib Nilotinib...
12 KB (821 words) - 02:57, 12 October 2024