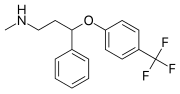
Drug expiration is the date after which a drug might not be suitable for use as manufactured. Consumers can determine the shelf life for a drug by checking...
12 KB (1,321 words) - 22:42, 6 October 2023
past their original expiration date. At least one drug worked 15 years after its expiration date. Joel Davis, a former FDA expiration-date compliance chief...
32 KB (3,375 words) - 21:55, 13 September 2024
guarantee the safety of a food or drug, and a product is not necessarily dangerous or ineffective after the expiration date. According to the United States...
36 KB (4,349 words) - 16:15, 13 October 2024
Shelf life (redirect from List of Food expiration)
the expiration date. The expiration date of pharmaceuticals specifies the date the manufacturer guarantees the full potency and safety of a drug. Most...
22 KB (2,633 words) - 17:41, 10 August 2024
Shelf Life Extension Program (category Product expiration)
Authorization on using a drug past its expiration date (which is legally an unapproved use of a drug), or by a "expiration dating extension authority"...
7 KB (671 words) - 02:28, 11 August 2024
by Oboli A.U and copied to all Directors at the agency, following the expiration of Prof Mojisola Adeyeye. She was appointed on 12 November 2022 by the...
17 KB (1,888 words) - 17:10, 30 January 2024
Expiration is an independent feature film, directed by Gavin Heffernan and released in 2003. The film stars Heffernan as Sam, a man who unwittingly walks...
2 KB (100 words) - 17:46, 1 September 2022
before the other. Foods and pharmaceutical drugs can be sold at a discounted price, and, near the expiration date, they can be destinated to humanitarian...
6 KB (658 words) - 03:07, 7 February 2024
Naratriptan (category Drugs with non-standard legal status)
expiring on July 7, 2010. In July 2010, in the wake of the patent expiration, several drug manufacturers, including Roxane Labs, Sandoz and Teva Pharmaceuticals...
11 KB (954 words) - 09:58, 10 March 2024
the drug. But even as the patent term nears expiration, pharmaceutical manufacturers employ several strategies to delay the entry of generic drugs to market...
96 KB (11,760 words) - 01:13, 2 September 2024
Limiting the Manufacture and Regulating the Distribution of Narcotic Drugs was a drug control treaty promulgated in Geneva on 13 July 1931 that entered into...
14 KB (807 words) - 13:28, 12 September 2024
Orlistat (redirect from Alli (drug))
2006. Retrieved 8 April 2007. "Drug Patent Expirations in June 2009". DrugPatentWatch.com, in "Drug Patent Expirations in June 2009". Biotech Blog. 1...
39 KB (3,466 words) - 10:08, 22 May 2024
Pharmaceutical industry (redirect from Drug companies)
discovers, develops, produces, and markets pharmaceutical goods for use as drugs that function by being administered to (or self-administered by) patients...
123 KB (12,947 words) - 11:17, 11 October 2024
Biopharmaceutical (redirect from Biological drug)
known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological...
30 KB (2,882 words) - 11:11, 23 July 2024
Obocell (category Anti-obesity drugs)
Empty | #1692607921". Worthpoint. Retrieved 2017-07-20. "The Myth Of Drug Expiration Dates". Retrieved 2017-07-19. "SML-164: Mr. Obocell". www.sebastianworld...
2 KB (236 words) - 20:13, 4 March 2024
Fluoxetine (redirect from Reconcile (drug))
(2 August 2001). "Drug Maker Is Set to Ship Generic Prozac". The New York Times. "Patent Expiration Dates for Common Brand-Name Drugs". Archived from the...
118 KB (11,722 words) - 01:15, 5 October 2024
International Opium Convention (redirect from Advisory Committee on Traffic in Opium and Other Dangerous Drugs)
Conference, on 23 January 1912, is considered as the first international drug control treaty. It was registered in League of Nations Treaty Series on January...
19 KB (1,192 words) - 13:23, 12 September 2024
expiration and, therefore, Bolar’s experiments had a business purpose. Bolar also argued that public policy in favor of availability of generic drugs...
5 KB (490 words) - 20:07, 18 September 2023
selling that particular drug in the market. This was a Patent Cliff. The abrupt drop in sales expected after the date of patent expiration can be estimated with...
3 KB (391 words) - 05:33, 4 August 2024
Pharmaceutical distribution (redirect from Drug supply chain)
Data Element – National Number, Expiration Date, Batch/Lot Number, Serial Number In 2008, China’s State Food and Drug Administration (CFDA) made serialization...
11 KB (1,189 words) - 11:15, 9 August 2024
Dolutegravir/lamivudine/tenofovir (category Chemical pages without DrugBank identifier)
tentatively approved in the United States as of 2019, full approval is pending expiration of the US patents on dolutegravir (Tivicay) and tenofovir disoproxil (Viread)...
9 KB (555 words) - 04:24, 2 October 2023
Substance abuse prevention (redirect from Drug prevention)
Substance abuse prevention, also known as drug abuse prevention, is a process that attempts to prevent the onset of substance use or limit the development...
31 KB (3,583 words) - 11:46, 3 September 2024
including Pseudomonas aeruginosa and Staphylococcus aureus. Following expiration of the Bristol-Myers Squibb patent,[when?] cefepime became available as...
8 KB (600 words) - 20:51, 10 October 2024
of a drug under the Hatch-Waxman Act, the generic manufacturer must certify that they will not launch their generic until after the expiration of the...
5 KB (588 words) - 18:41, 11 March 2023
Amlodipine (category Drugs with non-standard legal status)
elimination. 20-25% of the drug is excreted in the faeces. Pfizer's patent protection on Norvasc lasted until 2007; total patent expiration occurred later in 2007...
45 KB (4,043 words) - 00:40, 30 September 2024
patent expiration to sell a copy . This system is said to aim for a balance between gaining public access to generic drugs and encouraging drug research...
41 KB (3,595 words) - 23:33, 2 October 2024
General anaesthetic (redirect from Sleeping drug)
serves as a large, slowly-filling reservoir for the drug. Inhaled anesthetics are eliminated via expiration, following diffusion into the lungs. This process...
23 KB (2,715 words) - 19:11, 2 September 2024
Escitalopram (category Drugs with non-standard legal status)
relative to the generic mixture of isomers of citalopram, prior to the expiration of the escitalopram patent in 2012, which led to charges of evergreening...
58 KB (5,390 words) - 03:24, 10 October 2024
Montelukast (category Multiple chemicals in Infobox drug)
2023 at the Wayback Machine "Drugs covered by patent 5,565,473. Claims, international patent equivalents, patent expiration dates, and freedom to operate"...
27 KB (2,287 words) - 07:53, 3 October 2024
2017/18. Drugs with sales above $5 billion in 2015 included: For the fourth quarter of 2013, the largest selling drugs were: List of drugs Lists about...
29 KB (281 words) - 06:29, 12 August 2024