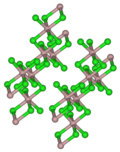
Dysprosium(III) chloride (DyCl3), also known as dysprosium trichloride, is a compound of dysprosium and chlorine. It is a white to yellow solid which...
6 KB (411 words) - 00:58, 29 September 2024
Dysprosium chloride may refer to: Dysprosium(II) chloride (dysprosium dichloride), DyCl2 Dysprosium(III) chloride (dysprosium trichloride), DyCl3 This...
500 bytes (51 words) - 01:30, 25 December 2015
phosphors, lasers, dysprosium metal halide lamps, and as a Faraday rotator. It can react with acids to produce the corresponding dysprosium(III) salts: Dy2O3...
3 KB (153 words) - 17:25, 1 August 2024
Dysprosium(II) chloride (DyCl2), also known as dysprosium dichloride, is an ionic chemical compound of dysprosium and chlorine. This salt is a reduced...
4 KB (369 words) - 10:31, 29 December 2023
Dysprosium(III) selenide can be prepared by reacting dysprosium(III) oxide or dysprosium(III) chloride with hydrogen selenide: Dy2O3 + 3 H2Se → Dy2Se3 +...
2 KB (148 words) - 17:51, 21 January 2024
DyF3. Dysprosium(III) fluoride can be produced by mixing dysprosium(III) chloride or dysprosium(III) carbonate into 40% hydrofluoric acid. DyCl3 + 3 HF →...
3 KB (269 words) - 05:05, 4 February 2024
Soluble dysprosium salts, such as dysprosium chloride and dysprosium nitrate are mildly toxic when ingested. Based on the toxicity of dysprosium chloride to...
37 KB (4,332 words) - 12:03, 15 September 2024
Terbium(III) chloride (TbCl3) is a chemical compound. In the solid state TbCl3 has the YCl3 layer structure. Terbium(III) chloride frequently forms a hexahydrate...
5 KB (371 words) - 17:32, 1 August 2024
Holmium(III) chloride is the inorganic compound with the formula HoCl3. It is a common salt but is mainly used in research. It can be used to produce...
4 KB (303 words) - 11:23, 8 July 2023
Dysprosium(III) chloride – DyCl3 Erbium(III) chloride – ErCl3 Europium(II) chloride – EuCl2 Europium(III) chloride – EuCl3 Gadolinium(III) chloride –...
119 KB (8,735 words) - 14:26, 16 September 2024
DyCl2 dysprosium(II) chloride 13767–31–2 DyCl3 dysprosium(III) chloride 10025–74–8 DyF3 dysprosium(III) fluoride 13569–80–7 DyH3 dysprosium(III) hydride...
139 KB (120 words) - 17:07, 15 July 2024
ion DyBr3 dysprosium(III) bromide 14456-48-5 DyCl2 dysprosium(II) chloride 13767-31-2 DyCl3 dysprosium(III) chloride 10025-74-8 DySi2 dysprosium(II) silicide...
183 KB (107 words) - 22:11, 6 September 2024
Dysprosium(II) iodide is an iodide of dysprosium with the chemical formula DyI2. Dysprosium(II) iodide can be produced by reducing dysprosium(III) iodide...
3 KB (284 words) - 08:40, 16 January 2024
Upham, H. C. (1966). "Pharmacology and toxicology of dysprosium, holmium, and erbium chlorides". Toxicology and Applied Pharmacology. 8 (1): 37–43. Bibcode:1966ToxAP...
36 KB (4,143 words) - 22:51, 11 September 2024
It is ferromagnetic, like gadolinium(III) nitride, terbium(III) nitride and dysprosium(III) nitride. Neodymium(III) nitride is not usually stoichiometric...
5 KB (316 words) - 17:11, 1 February 2024
Holmium(III) oxide, or holmium oxide is a chemical compound of the rare-earth element holmium and oxygen with the formula Ho2O3. Together with dysprosium(III)...
14 KB (1,188 words) - 19:39, 21 August 2024
Lanthanide trifluoromethanesulfonates (redirect from Dysprosium(III) triflate)
acid catalysts. These catalysts function similarly to aluminium chloride or ferric chloride, but they are water-tolerant (stable in water). Commonly written...
8 KB (924 words) - 07:50, 18 October 2023
the "interpenetrating primitive cubic" structure, also called a "caesium chloride" or B2 structure. This structure is often confused for a body-centered...
49 KB (3,620 words) - 23:33, 27 September 2024
Dysprosium iodate is an inorganic compound with the chemical formula Dy(IO3)3. It can be obtained by the reaction of dysprosium nitrate or dysprosium...
2 KB (173 words) - 15:52, 2 May 2024
Chromium (redirect from Chromium(III))
oxidize to chromium(III) derivatives in air. Water-stable chromium(II) chloride CrCl 2 that can be made by reducing chromium(III) chloride with zinc. The resulting...
109 KB (11,824 words) - 01:53, 10 September 2024
Nitrate chlorides are mixed anion compounds that contain both nitrate (NO3−) and chloride (Cl−) ions. Various compounds are known, including amino acid...
63 KB (3,736 words) - 14:02, 19 September 2024
M. N. Bochkarev, S. Dechert, H. Schumann: Reactions of neodymium(II), dysprosium(II), and thulium(II) diiodides with cyclopentadiene In: Russian Chemical...
11 KB (905 words) - 20:24, 3 January 2024
Upham, H. C. (1966). "Pharmacology and toxicology of dysprosium, holmium, and erbium chlorides". Toxicology and Applied Pharmacology. 8 (1): 37–43. Bibcode:1966ToxAP...
34 KB (4,095 words) - 04:24, 28 September 2024
yttrium forms trihalides such as yttrium(III) fluoride (YF 3), yttrium(III) chloride (YCl 3), and yttrium(III) bromide (YBr 3) at temperatures above roughly...
58 KB (6,666 words) - 17:30, 28 September 2024
formation of silver chloride due to long-term immersion in salt water, as well as reaction with nitrate ions or oxygen. Fresh silver chloride is pale yellow...
94 KB (11,248 words) - 04:16, 23 September 2024
tetrakis(methylammonium) hexachloroferrate(III) chloride (I) and tetrakis(hexamethylenediammonium) hexachloroferrate(III) tetrachloroferrate(III) tetrachloride (II)". Inorganica...
150 KB (17,056 words) - 12:22, 24 August 2024
derivatives. Au(III) (referred to as auric) is a common oxidation state, and is illustrated by gold(III) chloride, Au2Cl6. The gold atom centers in Au(III) complexes...
142 KB (15,991 words) - 10:04, 28 September 2024
beyond that for chlorine to chloride, +1.36 V. Consequently, cobalt(III) chloride would spontaneously reduce to cobalt(II) chloride and chlorine. Because the...
116 KB (11,828 words) - 10:09, 19 September 2024
result from exposure to water-soluble forms of mercury (such as mercuric chloride or methylmercury) either directly or through mechanisms of biomagnification...
119 KB (12,656 words) - 01:37, 27 September 2024
metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since...
141 KB (13,796 words) - 01:03, 27 September 2024