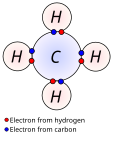
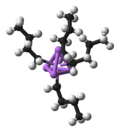
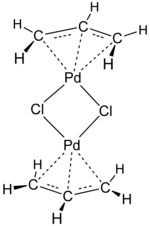
generally exist as free species. Two methods of electron counting are "neutral counting" and "ionic counting". Both approaches give the same result (and can...
14 KB (1,629 words) - 12:44, 22 September 2024
The d electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition...
14 KB (1,701 words) - 03:27, 4 October 2023
Lewis structure (redirect from Electron Dot Structure)
the need for electron counting: the atoms are drawn showing the valence electrons; bonds are then formed by pairing up valence electrons of the atoms...
16 KB (2,140 words) - 05:43, 27 October 2024
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such...
20 KB (2,190 words) - 16:15, 15 October 2024
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond...
24 KB (2,333 words) - 14:12, 27 October 2024
Octet rule (section Three-electron bonds)
strictly obey the 18-electron rule and the valence electron count can vary between 12 and 18. Lewis structure Electron counting Housecroft, Catherine...
22 KB (2,870 words) - 20:18, 28 October 2024
fulfill the 18-electron rule when one considers only those valence electrons, which occupy metal–ligand bonding orbitals. Electron counting – Formalism used...
17 KB (1,923 words) - 02:05, 19 June 2024
related to Electron shell diagrams. Periodic table (electron configurations) Electron counting 18-electron rule Core charge Re: Why do electron shells have...
28 KB (2,778 words) - 20:59, 1 November 2024
VSEPR theory (redirect from Valence shell electron pair repulsion)
to resonance. The "AXE method" of electron counting is commonly used when applying the VSEPR theory. The electron pairs around a central atom are represented...
45 KB (4,038 words) - 12:11, 1 November 2024
(1928). "Elektronenzählrohr zur Messung schwächster Aktivitäten (Electron counting tube for the measurement of the weakest radioactivities)". Die Naturwissenschaften...
10 KB (944 words) - 07:33, 20 August 2024
Jemmis mno rules (section Electron-counting rules)
one polyhedron. Electron-counting rules are used to predict the preferred electron count for molecules. The octet rule, the 18-electron rule, and Hückel's...
14 KB (1,581 words) - 12:51, 25 September 2023
one electron to the metal and accept one electron from the metal when using the neutral ligand method of electron counting, or donate two electrons to...
6 KB (734 words) - 09:09, 9 January 2024
The electron ( e− , or β− in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first...
154 KB (15,572 words) - 12:22, 9 November 2024
Geiger counter (category Counting instruments)
(1928), "Elektronenzählrohr zur Messung schwächster Aktivitäten" (Electron counting tube for the measurement of the weakest radioactivities), Die Naturwissenschaften...
24 KB (2,875 words) - 03:40, 9 October 2024
(1928). "Elektronenzählrohr zur Messung schwächster Aktivitäten" [Electron counting tube for measurement of weakest radioactivities]. Die Naturwissenschaften...
32 KB (4,296 words) - 02:52, 24 May 2024
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure)...
60 KB (6,147 words) - 15:47, 29 October 2024
single electron counts ("counting mode"). These Direct Electron Detectors are available from Gatan, FEI, Quantum Detectors and Direct Electron. A TEM...
118 KB (15,058 words) - 13:31, 17 October 2024
subsequently, detected by an electron counting instrument such as a charge-coupled device camera. Specifically, after the electron pulse diffracts from the...
11 KB (1,215 words) - 20:28, 24 August 2024
ligand that donates 2 electrons to the complex, and therefore lacks anionic or cationic properties that would affect the electron count of the complex. For...
8 KB (1,036 words) - 17:52, 28 March 2024
As in other areas of chemistry, electron counting is useful for organizing organometallic chemistry. The 18-electron rule is helpful in predicting the...
31 KB (3,157 words) - 20:03, 24 September 2024
Co(NO)(CO)3 and Ni(CO)4 illustrate the analogy between NO+ and CO. In an electron-counting sense, two linear NO ligands are equivalent to three CO groups. This...
17 KB (2,019 words) - 15:22, 2 September 2024
Olympicene (section Electron counting)
and David Mazziotti of the University of Chicago. Olympicene has 18 pi electrons in its ring system; as it is a flat molecule, this makes it an aromatic...
8 KB (852 words) - 05:53, 9 February 2024
total of 24 valence electrons, or 2e/Mo-Mo vector. More electron-deficient derivatives such as Ta6Cl184− have fewer d-electrons. For example, the naked...
10 KB (1,145 words) - 06:59, 23 July 2024
individual electron counts can be interpreted. More recently, direct electron detectors have been successfully used in both linear and counting modes. In...
20 KB (2,142 words) - 04:46, 18 August 2024
sometimes be not as strict, sometimes requiring identity of the total electron count and with it the entire electronic configuration. More usually, definitions...
5 KB (436 words) - 14:21, 10 March 2024
Hexaphosphabenzene (section Electron count)
is taken as a 10π electron donor, a 32 valence electron count may be obtained. In most triple-decker complexes with an electron count ranging from 26 to...
34 KB (3,519 words) - 04:22, 20 September 2024
chemically in a way that will allow them to have a closed shell of electrons, in this new counting scheme.[citation needed] Certain aluminum clusters have superatom...
17 KB (1,783 words) - 06:51, 23 July 2024
obey a single set of generalized selection rules, depending on the electron count and topology of the orbital interactions. The key concept of orbital...
93 KB (11,923 words) - 14:39, 12 August 2024
approach that does not invoke d orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal...
53 KB (7,366 words) - 22:03, 6 November 2024
scheme, serving as a 3e– donor using neutral electron counting and 4e– donor using ionic electron counting. More common are complexes with allyl and other...
12 KB (1,344 words) - 06:32, 27 April 2024