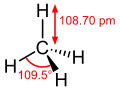In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics...
8 KB (853 words) - 13:20, 9 November 2024
exist for the halogenation of organic compounds, including free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition...
10 KB (1,112 words) - 15:24, 12 June 2024
substitution, for instance free-radical halogenation and autoxidation. Free-radical addition reactions Intramolecular free radical reactions (substitution...
5 KB (677 words) - 00:02, 31 January 2024
similar reactivity. The other radical reactions: radical substitution radical polymerization free-radical halogenation McMurry, John; Emeritus, Professor...
10 KB (934 words) - 02:20, 7 October 2024
This is because a new radical is created, able to participate in secondary reactions. In free radical halogenation reactions, radical substitution takes...
3 KB (351 words) - 10:21, 21 December 2024
radical reactions. One such example is the homolysis of halogens, which occurs under light and serves as the driving force for radical halogenation reactions...
40 KB (4,613 words) - 23:02, 27 November 2024
Methane (section Methane radical reactions)
mechanism for this process is called free radical halogenation. It is initiated when UV light or some other radical initiator (like peroxides) produces...
91 KB (8,881 words) - 06:30, 22 December 2024
ones are alkanes and alkenes. Alkanes react with halogens by free radical halogenation. In this reaction a hydrogen atom is removed from the alkane,...
20 KB (2,413 words) - 06:14, 8 September 2024
perceived RSP found in older organic chemistry textbooks concerns the free radical halogenation of simple alkanes. Whereas the relatively unreactive bromine reacts...
4 KB (561 words) - 00:17, 27 June 2024
synthesis Frankland–Duppa reaction Fráter–Seebach alkylation Free radical halogenation Freund reaction Friedel–Crafts acylation Friedel–Crafts alkylation...
37 KB (3,428 words) - 16:27, 11 November 2024
either methyl chloride (CH3Cl) or methane (CH4). At 400–500 °C, free radical halogenation occurs, converting these precursors to progressively more chlorinated...
61 KB (5,773 words) - 02:01, 21 December 2024
Alkane (section Free radical reactions)
degradation. Free radicals, molecules with unpaired electrons, play a large role in most reactions of alkanes. Free radical halogenation reactions occur...
61 KB (6,866 words) - 04:18, 14 December 2024
involve chiefly free radical reactions. Ethane can react with the halogens, especially chlorine and bromine, by free-radical halogenation. This reaction...
29 KB (2,859 words) - 00:52, 22 November 2024
by the bromination of toluene under conditions suitable for a free radical halogenation: The structure has been examined by electron diffraction. Benzyl...
5 KB (308 words) - 20:33, 23 February 2024
the benzylic radical. For related reasons, benzylic substituents exhibit enhanced reactivity, as in oxidation, free radical halogenation, or hydrogenolysis...
15 KB (1,603 words) - 15:22, 22 October 2024
this chain reaction resembles free radical halogenation, in which the peroxide promotes formation of the bromine radical. However, this process is restricted...
6 KB (788 words) - 07:43, 16 November 2023
those typical of other saturated hydrocarbons, combustion and free radical halogenation. Work in 2009 on alkane functionalisation, using peroxides such...
7 KB (478 words) - 13:02, 5 December 2024
consumption of ozone and the enhancement of halogens. Arctic haze Free radical halogenation Tropospheric ozone Frost flower (sea ice) Cao, Le; Platt, Ulrich;...
9 KB (997 words) - 05:12, 26 May 2024
with a single unpaired electron. This is the initiation stage of free radical halogenation. Heterolytic bond cleavage is a process where the electron pair...
18 KB (2,084 words) - 03:50, 17 October 2024
Wohl–Ziegler bromination (category Free radical reactions)
and promote a greater degree of brominated product formation. Free-radical halogenation Alfred Wohl (1919). "Bromierung ungesättigter Verbindungen mit...
10 KB (1,161 words) - 10:59, 29 April 2022
Substitution reaction (section Radical substitution)
reaction is halogenation. When chlorine gas (Cl2) is irradiated, some of the molecules are split into two chlorine radicals (Cl•), whose free electrons...
12 KB (1,456 words) - 13:55, 26 August 2024
antiplatelet agent working as a thromboxane A2 synthesis inhibitor. The free-radical halogenation of ethyl 4-methylcinnamate (1) with N-bromosuccinimide in the...
4 KB (184 words) - 15:52, 4 July 2024
heterocycle with a P-C bridge. It can also undergo halogenation and reaction with elemental sulfur. Phosphorus radicals are commonly characterized by EPR/ESR to...
43 KB (4,298 words) - 01:14, 14 December 2024
H2C=C(CH3)CH2Cl + C6H6 → C6H5C(CH3)2CH2Cl It can also be prepared by free radical halogenation of tert-butylbenzene, using various chlorine donors. Neophyl chloride...
5 KB (330 words) - 14:47, 10 August 2024
4-aminomethylpiperidine-1-carboxylate hydrochloride [359629-16-6] (2). The free-radical halogenation of ethyl o-toluate [87-24-1] (3) led to ethyl 2-(bromomethyl)benzoate...
8 KB (614 words) - 23:55, 14 November 2024
Hunsdiecker reaction (category Free radical reactions)
an organic halide. It is an example of both a decarboxylation and a halogenation reaction as the product has one fewer carbon atoms than the starting...
15 KB (1,388 words) - 09:41, 28 November 2024
toluene, the alkyl positions tend to be halogenated by free radical conditions, whereas ring halogenation is favored in the presence of Lewis acids. The decolouration...
16 KB (1,804 words) - 21:38, 3 June 2024
used in a variety of halogenation reactions. This compound is commercially available. It is prepared by exhaustive free radical chlorination of dimethyl...
8 KB (648 words) - 11:46, 2 December 2024
3-chloropentanes, as well as more highly chlorinated derivatives. Other radical halogenations can also occur. Pentane is produced by fractional distillation of...
12 KB (1,006 words) - 20:24, 2 November 2024
electrophilic halogen sources without transition-metal catalysts to give ipso halogenation product under mild conditions. For the bromination (with NBS) and iodination...
13 KB (1,337 words) - 23:38, 28 August 2023