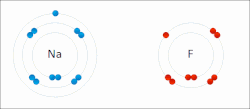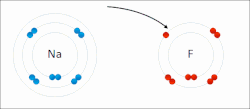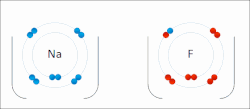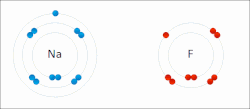
In chemistry, the lattice energy is the energy change (released) upon formation of one mole of a crystalline compound from its infinitely separated constituents...
14 KB (1,828 words) - 06:10, 26 May 2025
of calculating the lattice energy of a crystalline ionic compound. In 1918 Max Born and Alfred Landé proposed that the lattice energy could be derived from...
5 KB (839 words) - 17:28, 14 May 2025
effect on their bond energy. The extent of this effect is described by the compound's lattice energy, where a more negative lattice energy corresponds to a...
10 KB (1,317 words) - 12:02, 25 May 2025
a means of calculating lattice energy (or more precisely enthalpy), which cannot otherwise be measured directly. The lattice enthalpy is the enthalpy...
7 KB (941 words) - 22:38, 25 March 2025
Brillouin zone (redirect from Reciprocal lattice primitive cell)
In the same way the Bravais lattice is divided up into Wigner–Seitz cells in the real lattice, the reciprocal lattice is broken up into Brillouin zones...
11 KB (640 words) - 06:31, 24 September 2024
other releases (lattice) energy and, thus, lowers the overall energy of the system. Ionic bonding will occur only if the overall energy change for the...
18 KB (2,340 words) - 19:04, 15 June 2025
crystalline solid comprises the hydration energy. If the hydration energy is greater than the lattice energy, then the enthalpy of solution is negative...
3 KB (342 words) - 19:54, 15 February 2025
The Kapustinskii equation calculates the lattice energy UL for an ionic crystal, which is experimentally difficult to determine. It is named after Anatoli...
4 KB (430 words) - 20:13, 4 February 2025
bonds for one mole of an ionic solid under standard conditions is the lattice energy. The Madelung constant allows for the calculation of the electric potential...
14 KB (1,711 words) - 22:53, 25 March 2025
amount of heat (known as the lattice energy) to the surroundings. Although heat is "wasted" energy for a specific energy transfer (see: waste heat), it...
60 KB (7,503 words) - 22:24, 27 June 2025
a reasonable form is assumed for the additional repulsive energy, the total lattice energy can be modelled using the Born–Landé equation, the Born–Mayer...
63 KB (6,979 words) - 02:34, 2 June 2025
electron diffraction as well as the energies of electrons in a solid. It emerges from the Fourier transform of the lattice associated with the arrangement...
33 KB (5,497 words) - 02:05, 20 June 2025
Solvation (redirect from Solvation energy)
hydration. Solubility of solid compounds depends on a competition between lattice energy and solvation, including entropy effects related to changes in the solvent...
19 KB (2,405 words) - 05:12, 24 June 2025
Band gap (redirect from Band gap energy)
the lattice phonons and the free electrons and holes will also affect the band gap to a smaller extent. The relationship between band gap energy and temperature...
26 KB (2,785 words) - 07:38, 24 May 2025
Ising model (redirect from Spin lattice)
maximum difference in energy would be 4J. Let c represent the lattice coordination number; the number of nearest neighbors that any lattice site has. We assume...
88 KB (13,240 words) - 10:22, 30 June 2025
Lattice QCD is a well-established non-perturbative approach to solving the quantum chromodynamics (QCD) theory of quarks and gluons. It is a lattice gauge...
15 KB (1,870 words) - 20:02, 19 June 2025
property Enthalpy of mixing Heat of dilution Heat of melting Hydration energy Lattice energy Law of dilution Solvation Thermodynamic activity Solubility equilibrium...
5 KB (681 words) - 06:11, 18 February 2025
assumed. Δ H sublimation = − U lattice energy − 2 R T {\displaystyle \Delta H_{\text{sublimation}}=-U_{\text{lattice energy}}-2RT} Dye-sub printing is a...
23 KB (2,429 words) - 15:34, 25 June 2025
concept describing the energy released by adding an electron to a neutral atom or molecule. Lattice energy, a measure of the energy released when ions are...
51 KB (5,844 words) - 16:50, 28 June 2025
Born, proposed the Born–Haber cycle as a method for evaluating the lattice energy of an ionic solid. Haber, a known German nationalist, is also considered...
67 KB (7,303 words) - 15:47, 12 May 2025
The Born–Mayer equation is an equation that is used to calculate the lattice energy of a crystalline ionic compound. It is a refinement of the Born–Landé...
1 KB (152 words) - 15:23, 22 October 2023
acquire Lattice Energy from Origin Energy for $1.585 billion, significantly increasing the company's operations portfolio. Beach Energy Ltd. "Beach Energy Annual...
5 KB (362 words) - 14:03, 7 November 2024
2017, Origin sold its subsidiary Lattice Energy and its conventional upstream oil and gas business to Beach Energy for $1,585 million. Australia Pacific...
30 KB (2,178 words) - 02:12, 29 March 2025
dissociation energies for common organic compounds are shown below. Bond energy Electronegativity Ionization energy Electron affinity Lattice energy IUPAC,...
24 KB (2,441 words) - 16:09, 24 May 2025
experimentally, but the lattice energy cannot be measured directly. The equation is therefore rearranged to evaluate the lattice energy: − U L = Δ H sub +...
29 KB (1,879 words) - 21:49, 30 April 2025
Alkali metal (section First ionisation energy)
all the energy released from the formation of an alkali metal nitride is from the lattice energy of the alkali metal nitride. The lattice energy is maximised...
225 KB (24,164 words) - 20:18, 19 June 2025
Electronic band structure (redirect from Energy band)
with different energies.: 117–122 Similarly, if a large number N of identical atoms come together to form a solid, such as a crystal lattice, the atoms'...
37 KB (4,835 words) - 02:03, 12 May 2025
positively-charged Na+ and negatively-charged Cl− ions, which releases a 8.12 eV lattice energy. By contrast, any further electrons removed from Na would reside in...
23 KB (2,861 words) - 21:13, 19 June 2025
higher energy, non-equilibrium state to thermodynamic equilibrium with its surroundings (the "lattice"). It is characterized by the spin–lattice relaxation...
12 KB (1,602 words) - 18:13, 27 May 2024
dimethylglyoxime, (dmgH2): in this case the lattice energy of the solid is not particularly large, but it greatly exceeds the energy of solvation of the molecule Ni(dmgH)2...
44 KB (6,727 words) - 11:35, 24 June 2025