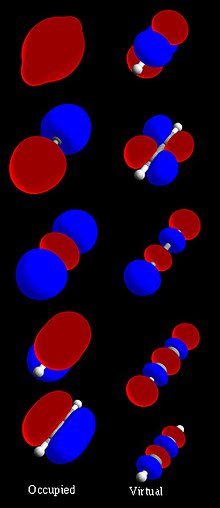
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory...
39 KB (4,968 words) - 12:08, 22 September 2024
region. The terms atomic orbital and molecular orbital were introduced by Robert S. Mulliken in 1932 to mean one-electron orbital wave functions. At an elementary...
35 KB (4,390 words) - 11:31, 6 June 2024
transition of electrons moving from one orbital at a lower energy to a higher energy orbital. The molecular orbital diagram for the final state describes the...
23 KB (3,018 words) - 12:43, 22 October 2024
atomic orbital is combined in the term. The coefficients are the weights of the contributions of the n atomic orbitals to the molecular orbital. The Hartree–Fock...
6 KB (781 words) - 00:03, 6 April 2023
respect to those atoms. Antibonding orbitals are often labelled with an asterisk (*) on molecular orbital diagrams. In homonuclear diatomic molecules,...
7 KB (880 words) - 16:28, 11 November 2024
A diagram is a symbolic representation of information using visualization techniques. Diagrams have been used since prehistoric times on walls of caves...
16 KB (1,060 words) - 09:41, 12 November 2024
π* orbital of an electron-withdrawing group in the form of a molecular orbital diagram. The HOMO of a radical is singly-occupied hence the orbital is...
40 KB (4,613 words) - 17:41, 23 November 2024
is occupied by two electrons of the same spin, as shown in the molecular orbital diagram. The molecule, therefore, has two unpaired electrons and is in...
7 KB (814 words) - 22:54, 5 September 2023
molecules. By plotting the change in molecular orbital levels of a molecule as a function of geometrical change, Walsh diagrams explain why molecules are more...
15 KB (1,732 words) - 18:19, 4 November 2024
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies,...
33 KB (3,170 words) - 15:02, 7 November 2024
HOMO and LUMO (redirect from Highest occupied molecular orbital)
LUMO are types of molecular orbitals. The acronyms stand for highest occupied molecular orbital and lowest unoccupied molecular orbital, respectively. HOMO...
4 KB (402 words) - 06:52, 4 October 2024
linked by a single bond. The molecular orbital diagram of the peroxide dianion predicts a doubly occupied antibonding π* orbital and a bond order of 1. The...
9 KB (1,065 words) - 15:03, 9 November 2024
Non-bonding orbitals are often designated by the letter n in molecular orbital diagrams and electron transition notations. Non-bonding orbitals are the equivalent...
5 KB (660 words) - 20:31, 24 June 2023
Anti-periplanar (section Molecular orbitals)
the interaction between molecular orbitals. Anti-periplanar geometry will put a bonding orbital and an anti-bonding orbital approximately parallel to...
11 KB (980 words) - 07:49, 9 May 2024
states has its two valence electrons spin-paired in one π* orbital while the second π* orbital is empty. This state is referred to by the title term, singlet...
29 KB (2,967 words) - 22:27, 24 November 2024
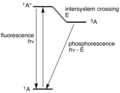
In molecular spectroscopy, a Jablonski diagram is a diagram that illustrates the electronic states and often the vibrational levels of a molecule, and...
4 KB (456 words) - 11:17, 14 September 2024
of orbitals. This adds up to a bond order of 2, meaning that there exists a double bond between the two carbon atoms. The molecular orbital diagram of...
7 KB (792 words) - 20:17, 7 October 2024
general d-orbital splitting diagram for square planar (D4h) transition metal complexes can be derived from the general octahedral (Oh) splitting diagram, in...
5 KB (451 words) - 04:50, 4 November 2023
two molecular fragments have similar frontier orbitals, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO)...
10 KB (1,091 words) - 07:59, 7 March 2024
a molecular orbital diagram. In the standard Diels–Alder, the electron rich diene has molecular orbitals that are higher in energy than the orbitals of...
26 KB (2,637 words) - 20:27, 12 July 2024
mixed to make bonding orbital 1b1 and antibonding orbital 2b1. The two remaining 2p orbitals are unmixed. While this simple MO diagram does not provide four...
15 KB (1,822 words) - 22:24, 10 November 2024
has symmetry group D2h, and its highest occupied molecular orbital (HOMO) is the bonding pi orbital which forms a basis for its irreducible representation...
48 KB (4,086 words) - 14:28, 10 November 2024
Computational chemistry (section Molecular mechanics)
sets are then used to describe molecular orbitals via the linear combination of atomic orbitals (LCAO) molecular orbital method ansatz. A common type of...
76 KB (8,349 words) - 22:36, 22 November 2024
In quantum mechanics, an atomic orbital (/ˈɔːrbɪtəl/) is a function describing the location and wave-like behavior of an electron in an atom. This function...
84 KB (10,942 words) - 01:33, 19 November 2024
Nitrogen (redirect from Molecular nitrogen)
very small and has a very similar radius to the 2s shell, facilitating orbital hybridisation. It also results in very large electrostatic forces of attraction...
105 KB (12,228 words) - 09:07, 17 November 2024
effects of spin-orbit coupling in metal to ligand charge transfer (MLCT) transitions. As shown in figure 1, the molecular orbital diagram of [(n-C4H9)4N]2Pt(CN)4...
29 KB (4,682 words) - 21:51, 30 March 2024
measurements of fundamental constants. Morse/Long-range potential Molecular orbital diagram § Dilithium Dilithium (Star Trek) Chemical Bonding, Mark J. Winter...
9 KB (700 words) - 21:48, 31 October 2024
states. The t2g orbital set holds the single electron and has a 2T2g state energy of -4Dq. When that electron is promoted to an eg orbital, it is excited...
21 KB (2,288 words) - 18:18, 4 November 2024
chemistry, the bonding orbital is used in molecular orbital (MO) theory to describe the attractive interactions between the atomic orbitals of two or more atoms...
9 KB (1,093 words) - 22:34, 24 September 2022
Woodward–Hoffmann rules (redirect from Conservation of orbital symmetry)
experimental evidence and molecular orbital analysis as follows: In an open-chain system containing 4n π electrons, the orbital symmetry of the highest...
93 KB (11,923 words) - 14:39, 12 August 2024