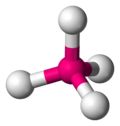
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies,...
33 KB (3,164 words) - 10:14, 24 April 2024
Hybridization (or hybridisation) may refer to: Hybridization (biology), the process of combining different varieties of organisms to create a hybrid Orbital hybridization...
1 KB (175 words) - 02:42, 28 February 2022
Bent's rule (section Nonbonding orbitals)
Chemistry portal Molecular orbital theory Orbital hybridisation Molecular geometry Linear combination of atomic orbitals Weinhold, F.; Landis, C. L....
38 KB (4,243 words) - 07:47, 11 June 2024
properties of the peripheral atoms (X). Other cases also experience orbital hybridisation, but in different degrees. AX2E1 molecules, such as SnCl2, have...
3 KB (328 words) - 17:23, 26 January 2024
state and the most populous city of Brazil sp orbitals, in physics, an instance of atomic orbital hybridisation Self-propelled (disambiguation) Soft-point...
5 KB (672 words) - 19:27, 26 July 2024
contributions to the theory of the chemical bond include the concept of orbital hybridisation and the first accurate scale of electronegativities of the elements...
141 KB (13,984 words) - 06:19, 15 August 2024
representing a large degree of strain.[citation needed] AXE method Orbital hybridisation Alger, Nick. "Angle Between 2 Legs of a Tetrahedron". Archived from...
11 KB (1,197 words) - 20:00, 21 July 2024
new electron in a 2p orbital; carbon (1s2 2s2 2p2) fills a second 2p orbital; and with nitrogen (1s2 2s2 2p3) all three 2p orbitals become singly occupied...
252 KB (27,200 words) - 06:27, 15 August 2024
explained in terms of orbital hybridisation. In ethylene each carbon atom has three sp2 orbitals and one p-orbital. The three sp2 orbitals lie in a plane with...
8 KB (933 words) - 06:40, 19 May 2024
form a chemical bond, the atomic orbitals of each atom are said to combine in a process called orbital hybridisation. The two most common types of bonds...
23 KB (2,310 words) - 14:26, 4 May 2024
small and has a very similar radius to the 2s shell, facilitating orbital hybridisation. It also results in very large electrostatic forces of attraction...
105 KB (12,223 words) - 08:00, 14 August 2024
adopting sp orbital hybridisation on both atoms, featuring one σ bond (between one sp orbital on each atom) and one π bond (between aligned p orbitals on each...
12 KB (1,018 words) - 18:09, 30 June 2024
Live Longer and Feel Better (1986) Concepts Valence bond theory Orbital hybridisation Resonance Pauling Electronegativity Scale Pauling's rules Founded...
22 KB (2,337 words) - 14:30, 27 April 2024
is the only orbital that is completely unscreened from the nucleus, and there is no other orbital of similar energy for it to hybridise with (it also...
8 KB (1,068 words) - 06:10, 23 July 2024
The term may refer to either an atomic orbital or a molecular orbital. orbital hybridisation order of reaction organic acid Any organic compound with acidic...
171 KB (18,187 words) - 16:16, 23 May 2024
valence bond theory, these molecules can be described as adopting sp2 orbital hybridisation, featuring one sigma and two pi bonds. The corresponding ground...
5 KB (607 words) - 18:31, 2 April 2024
Live Longer and Feel Better (1986) Concepts Valence bond theory Orbital hybridisation Resonance Pauling Electronegativity Scale Pauling's rules Founded...
4 KB (113 words) - 22:15, 17 October 2023
The carbon atoms in this form are each linear in geometry with sp orbital hybridisation. The estimated length of the bonds is 120.7 pm (triple) and 137...
25 KB (2,821 words) - 05:43, 13 August 2024
hypervalence in terms of d-orbital hybridisation, with the energy penalty of promoting electrons into the higher energy orbitals being off-set by the stabilisation...
61 KB (6,943 words) - 12:55, 25 July 2024
SP3 may refer to: sp3 hybrids, a type of orbital hybridisation Sp3 transcription factor, a protein and the gene which encodes it Savoia-Pomilio SP.3,...
768 bytes (153 words) - 10:22, 14 August 2019
cation is formed the central carbon is rehybridised from sp3 to sp2 Orbital hybridisation. This causes the atoms to exhibit a trigonal planar arrangement...
4 KB (423 words) - 06:21, 24 September 2020
magnetoresistance Giant magnetoresistance Molecular electronics Orbital hybridisation Cornia, Andrea; Seneor, Pierre (25 April 2017). "Spintronics: The...
22 KB (2,676 words) - 09:16, 2 January 2024
Isovalent hybridization (redirect from Isovalent hybridisation)
hybrids of s and p orbitals, this is the coefficient ( λ ) {\displaystyle (\lambda )} multiplying the p orbital when the hybrid orbital is written in the...
7 KB (1,049 words) - 01:09, 5 February 2021
hydride is the simplest transition metal molecule that displays sd3 orbital hybridisation. Breisacher, Peter; Siegel, Bernard (5 June 1963). "Formation of...
5 KB (476 words) - 17:56, 2 January 2024
could be classified as, for example, a 5-exo-trig. Baldwin discovered that orbital overlap requirements for the formation of bonds favour only certain combinations...
9 KB (859 words) - 13:26, 15 August 2024
orbital (e.g. C–H or C–C) with an adjacent unpopulated non-bonding p or antibonding σ* or π* orbitals to give a pair of extended molecular orbitals....
20 KB (2,394 words) - 12:04, 1 May 2024
sp2-hybridized orbital and a p-orbital that is not involved in the hybridization. A triple bond is formed with an sp-hybridized orbital and two p-orbitals from...
9 KB (1,043 words) - 22:45, 2 February 2024
only when the occupied lone pair orbital of the nucleophile donates electrons to the unfilled σ* antibonding orbital between the central carbon and the...
21 KB (2,554 words) - 20:30, 22 July 2024
explained. In general, the result of an orbital operator acting on vibronic states can be replaced by an effective orbital operator acting on purely electronic...
61 KB (8,002 words) - 17:53, 17 August 2024
in some melanistic examples. These are due to captive breeding and hybridisation between subspecies and with the green pheasant, reinforced by continual...
60 KB (5,608 words) - 20:44, 23 June 2024