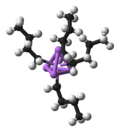
Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon (C) to vanadium (V) chemical bond. Organovanadium compounds...
6 KB (678 words) - 17:36, 27 April 2024
organoscandium chemistry, organotitanium chemistry, organovanadium chemistry, organochromium chemistry, organomanganese chemistry, organoiron chemistry, organocobalt...
31 KB (3,157 words) - 20:03, 24 September 2024
states. Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon (C) to vanadium (V) chemical bond. Organovanadium compounds...
16 KB (1,842 words) - 09:59, 21 June 2024
Industrial Chemistry. doi:10.1002/14356007.a27_367. ISBN 3-527-30673-0. Brauer, p. 1267 Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements...
17 KB (1,550 words) - 14:25, 4 October 2024
In chemistry, a vanadate is an anionic coordination complex of vanadium. Often vanadate refers to oxoanions of vanadium, most of which exist in its highest...
10 KB (1,169 words) - 22:53, 18 June 2024
experiencing a placebo effect. Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 1157. ISBN 978-0-08-022057-4...
10 KB (992 words) - 09:03, 22 November 2024
Vanadium oxytrichloride (section Organic chemistry)
Inorganic Chemistry. Academic Press. ISBN 978-0-12-352651-9. G. Brauer "Vanadium oxytrichloride" in Handbook of Preparative Inorganic Chemistry, 2nd Ed...
8 KB (646 words) - 20:00, 30 September 2023
forming adducts with pyridine and methylamine. It is used in organic chemistry as a catalyst for the epoxidation of allylic alcohols by tert-butyl hydroperoxide...
5 KB (468 words) - 17:16, 27 April 2024
Vanadocene dichloride (category Organovanadium compounds)
dichloride gives vanadocene, (C5H5)2V. Like titanocene dichloride, this organovanadium compound was investigated as a potential anticancer drug. It was conjectured...
4 KB (196 words) - 20:25, 6 August 2024
organometallic chemistry of vanadium is well–developed. Vanadocene dichloride is a versatile starting reagent and has applications in organic chemistry. Vanadium...
75 KB (8,566 words) - 05:49, 11 November 2024
Surv., vol. 1452, 1978. Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 982. ISBN 978-0-08-037941-8...
3 KB (216 words) - 19:31, 11 January 2023
Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic...
2 KB (214 words) - 07:34, 24 December 2023
Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1267. Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the...
3 KB (246 words) - 21:05, 15 October 2024
alkaloids.[citation needed] John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 4–40. ISBN 978-1138561632. Günter...
5 KB (354 words) - 11:47, 14 April 2024
Vanadium tetrachloride (category Reagents for organic chemistry)
Organovanadium(III) compounds V(C9H11)3...
5 KB (316 words) - 07:50, 7 February 2023
ISSN 1477-9234. PMID 23584576. "New Contributions to the Structural Chemistry of Vanadyl Orthophosphate VOPO4". edoc.mpg.de. Retrieved 2019-09-13. Girgsdies...
8 KB (736 words) - 01:24, 15 May 2024
modulus of approximately 380 GPa. Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 4–93, ISBN 0-8493-0594-2...
3 KB (263 words) - 23:32, 11 May 2024
Vanadium(III) sulfate is a reducing agent. Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 4–93, ISBN 0-8493-0594-2...
3 KB (186 words) - 16:07, 30 June 2024
[V(H2O)6]Cl2. Vanadium dichloride is used as a specialty reductant in organic chemistry. As an aqueous solution, it converts cyclohexylnitrate to cyclohexanone...
4 KB (236 words) - 14:38, 4 February 2023
(Cycloheptatrienyl)(cyclopentadienyl)vanadium (category Organovanadium compounds)
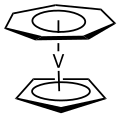
(Cycloheptatrienyl)(cyclopentadienyl)vanadium is an organovanadium compound with the formula V(C5H5)(C7H7). It is a purple, paramagnetic, sublimable solid...
3 KB (179 words) - 04:15, 29 December 2023
layers deposited by reactive magnetron sputtering". Journal of Materials Chemistry C. 4 (34): 7924–7938. doi:10.1039/C6TC02289H. ISSN 2050-7534. v t e...
5 KB (393 words) - 06:02, 3 March 2024
Oxydichloride". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. NY: Academic Press. p. 1263. Seifert, H. J.; Uebach, J. (1981)...
2 KB (167 words) - 14:19, 6 July 2023
with high specific capacity for Li ion batteries". Journal of Materials Chemistry A. 3 (41): 20508–20515. doi:10.1039/C5TA05434F. hdl:10637/7682. Davis...
3 KB (248 words) - 13:40, 13 August 2023
Trimesitylvanadium (category Organovanadium compounds)
Trimesitylvanadium (mesityl or Mes = 2,4,6-trimethylphenyl) is one of the organovanadium complexes with vanadium in an oxidation state of 3. This compound was...
14 KB (1,343 words) - 09:03, 13 July 2024
Y. Tyree (1964). "Chloro-Aquo Complexes of Vanadium(III)". Inorganic Chemistry. 3 (8): 1173–1176. doi:10.1021/ic50018a024. Yajima Akimasa; Matsuzaki...
17 KB (1,424 words) - 15:34, 12 July 2024
Vanadium(II) Sulfate Hexahydrate and Vanadium(II) Saccharinates". Inorganic Chemistry. 25 (19): 3423–3428. doi:10.1021/ic00239a021. Kranz, M. (1963). "Vanadium(II)...
4 KB (316 words) - 19:45, 27 October 2024
(November 1982). "The New Biochemistry of Vanadium". Comments on Inorganic Chemistry. 2 (1–2): 1–22. doi:10.1080/02603598208078107. Bard, Allen J. (1985)....
3 KB (304 words) - 15:25, 13 May 2024
Cyclopentadienylvanadium tetracarbonyl (category Organovanadium compounds)
Cyclopentadienylvanadium tetracarbonyl is the organovanadium compound with the formula (C5H5)V(CO)4. An orange, diamagnetic solid, it is the principal...
4 KB (279 words) - 23:10, 30 June 2024
(1979-12-01). "An N.M.R. study of the solution chemistry of vanadium pentafluoride". Journal of Fluorine Chemistry. 14 (6): 485–494. doi:10.1016/S0022-1139(00)82524-5...
9 KB (814 words) - 10:20, 27 August 2024
pubchem.ncbi.nlm.nih.gov. Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 1144–45. ISBN 978-0-08-022057-4...
26 KB (2,551 words) - 11:51, 24 August 2024