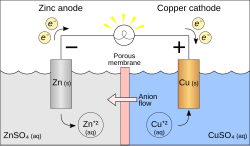
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and...
9 KB (998 words) - 05:51, 13 June 2025
electrochemical kinetics relating the rate of an electrochemical reaction to the overpotential. The Tafel equation was first deduced experimentally and was later shown...
10 KB (1,161 words) - 09:00, 17 March 2025
Concentration polarization (redirect from Concentration overpotential)
the term is used in this sense, it is equivalent to “concentration overpotential”. the changes in concentration (emergence of concentration gradients...
9 KB (1,191 words) - 18:05, 23 May 2024
water than predicted, and these are termed overpotentials. Experimentally it is known that overpotentials depend on the design of the cell and the nature...
51 KB (5,991 words) - 08:11, 27 June 2025
limitation, the relationship between the current density and the electrode overpotential is given by the Butler–Volmer equation: j t = j 0 ( exp ( α o f η...
25 KB (3,097 words) - 14:24, 13 August 2024
Electrolysis of water (section Overpotential)
needed] Electrolysis of pure water requires excess energy in the form of overpotential to overcome various activation barriers. Without the excess energy,...
77 KB (8,315 words) - 04:38, 30 June 2025
electrolytic process to overpotential. The exchange current density is the current in the absence of net electrolysis and at zero overpotential. The exchange current...
4 KB (470 words) - 19:28, 21 April 2025
transfer coefficient, dimensionless η {\displaystyle \eta } : activation overpotential (defined as η = E − E e q {\displaystyle \eta =E-E_{\rm {eq}}} ). The...
18 KB (3,205 words) - 08:23, 20 July 2024
reduction reaction (negative overpotential) and a positive current density for an oxidation reaction (positive overpotential). The sign of the current density...
3 KB (396 words) - 15:30, 20 October 2024
charge carriers and subsequent fast recombination, requiring a large overpotential to drive the reaction. Research has been focused on improving the water...
22 KB (2,156 words) - 03:11, 30 June 2025
water; in practice, losses from material internal resistance and the overpotential of the water splitting reaction increase the required bandgap energy...
34 KB (3,858 words) - 16:05, 22 May 2025
limited by incomplete discharge at the cathode, charging overpotential exceeding discharge overpotential, and component stability. During discharge of lithium–air...
21 KB (2,244 words) - 19:57, 19 June 2025
regulates the uniform electrochemical plating of Na metal, lowering overpotential and offering dendrite-free plating/stripping of Na metal over 100 h...
75 KB (7,826 words) - 20:57, 21 June 2025
anodes is made according to their oxygen evolution overpotential. Electrodes with low oxygen overpotential show an active behavior, as in the case of Platinum...
30 KB (3,728 words) - 07:04, 2 June 2025
the pH of the electrolyte (otherwise non-interacting). Assuming no overpotential, above the top line the out of equilibrium condition involves oxygen...
28 KB (4,311 words) - 23:01, 20 June 2025
A PEM electrolysis system's performance can be compared by plotting overpotential versus cell current density. This essentially results in a curve that...
23 KB (2,537 words) - 16:57, 18 June 2025
kinetics of this reaction are unfavorable, and there is also a bubble overpotential effect to consider, so that electrolysis of aqueous chloride solutions...
117 KB (13,095 words) - 22:59, 8 June 2025
constant, T the absolute temperature, and F is the Faraday constant. An overpotential occurs in the electrolysis of water. This means that the required cell...
4 KB (597 words) - 10:33, 29 July 2023
2017 cell designs, the charge overpotential is much higher than the discharge overpotential. Significant charge overpotential indicates the presence of secondary...
52 KB (5,758 words) - 18:45, 15 June 2025
high oxidation potential of the peroxodicarbonate anion, a high anodic overpotential is necessary. This is even more important if hydroxyl radicals are involved...
6 KB (593 words) - 21:26, 19 June 2025
electrode potential Electric potential Galvani potential Nernst equation Overpotential Potential difference (voltage) Standard electrode potential Table of...
7 KB (965 words) - 22:28, 7 June 2025
ozone electrolytically is typically unfavorable because of the high overpotential required to produce ozone as compared to oxygen. This is why ozone is...
150 KB (18,253 words) - 20:07, 25 June 2025
electrode, blocking these sites for the H+ adsorption and increase the overpotential for the hydrogen evolution reaction. One main challenge is to reduce...
27 KB (3,170 words) - 20:32, 22 May 2025
cocatalysts has been pivotal in enhancing charge separation and reducing overpotential, thereby improving the overall efficiency of the water-splitting reaction...
7 KB (775 words) - 13:58, 26 May 2025
1}\right){R_{\text{L}}}} This can also be expressed in terms of the Overpotential η and the current I: R i n t = η I {\displaystyle R_{int}={\frac {\eta...
6 KB (649 words) - 14:39, 21 January 2025
the production of hydrogen. An ideal WOC would operate rapidly at low overpotential, exhibit high stability and be of low cost, derived from nontoxic components...
15 KB (1,807 words) - 11:30, 6 May 2025
usually be reduced by various mechanisms, such as the development of overpotentials.(§ 25.12 "Working galvanic cells") Also, since chemical reactions occur...
22 KB (2,972 words) - 15:54, 21 May 2025
} : activation overpotential (defined as η = E − E e q {\displaystyle {\displaystyle \eta =E-E_{\rm {eq}}}} ). At high overpotentials, the Butler–Volmer...
30 KB (3,156 words) - 00:53, 25 May 2025
barrier for electron transfer. A theoretical description of polarization overpotential is in part described by the Butler–Volmer equation and Cottrell equation...
28 KB (3,491 words) - 22:59, 25 May 2025
(methanogenic archaea or methanogens) release enzymes that reduce the overpotential of a non-catalytic electrode (the cathode) so that it can produce hydrogen...
66 KB (5,863 words) - 01:09, 28 June 2025