Phosphorus pentasulfide is the inorganic compound with the formula P2S5 (empirical) or P4S10 (molecular). This yellow solid is the one of two phosphorus...
8 KB (652 words) - 04:24, 14 November 2024
significance, phosphorus pentasulfide (P4S10), which is made on a kiloton scale for the production of other organosulfur compounds, and phosphorus sesquisulfide...
7 KB (842 words) - 05:16, 14 November 2024
derived from white phosphorus include phosphorus pentasulfide and various metal phosphides. Although white phosphorus forms the tetrahedron, the simplest...
12 KB (1,162 words) - 10:09, 14 November 2024
participates: and in that of thiophene for instance the compound phosphorus pentasulfide: The acid catalyzed furan synthesis proceeds by protonation of...
15 KB (1,628 words) - 14:29, 8 June 2024
solution and reprecipitating arsenic pentasulfide at low temperature by addition of hydrochloric acid. Phosphorus pentasulfide with the formula P4S10, is a molecular...
4 KB (251 words) - 14:44, 19 July 2024
phosphorus chlorides and two phosphorus sulfides, phosphorus pentasulfide and phosphorus sesquisulfide. Organophosphorus compounds have many applications...
109 KB (12,801 words) - 13:56, 9 November 2024
acidification. It is, therefore, not analogous to the phosphorus(V) compound phosphorus pentasulfide. NIOSH Pocket Guide to Chemical Hazards. "#0036". National...
4 KB (261 words) - 20:26, 4 February 2024
structures. Those include phosphorus trioxide P4O6, arsenic trioxide As4O6, phosphorus pentoxide P4O10 = (PO)4O6, phosphorus pentasulfide P4S10 = (PS)4S6, and...
44 KB (4,343 words) - 03:11, 2 November 2024
is a colorless, distillable liquid. It is prepared by treating phosphorus pentasulfide with methanol: P2S5 + 4 CH3OH → 2 (CH3O)2PS2H + H2S Ammonium diethyl...
3 KB (124 words) - 13:10, 8 December 2022
trisulfide, As2S3, the mineral orpiment Arsenic pentasulfide, As2S5, similar structure to phosphorus pentasulfide (β-P2S5) Tetraarsenic tetrasulfide, As4S4...
796 bytes (75 words) - 04:59, 22 October 2024
Ferrophosphorus, reacted with sulfur or pyrite, is used for production of phosphorus pentasulfide. Ferrophosphorus can be used for production of lithium iron phosphate...
5 KB (486 words) - 15:24, 20 January 2024
H2S Many metal and nonmetal sulfides, e.g. aluminium sulfide, phosphorus pentasulfide, silicon disulfide liberate hydrogen sulfide upon exposure to water:...
76 KB (7,939 words) - 13:55, 16 November 2024
Lawesson's reagent (category Phosphorus heterocycles)
prepared in the laboratory by heating a mixture of anisole with phosphorus pentasulfide until the mixture is clear and no more hydrogen sulfide is formed...
9 KB (685 words) - 00:12, 29 October 2024
produced by the reaction of red or white phosphorus with sulfur. Excess sulfur gives phosphorus pentasulfide (P4S10). It is estimated that 150 ton/y were...
7 KB (572 words) - 06:14, 24 July 2024
Phosphorite (category Phosphorus)
fertilizers. Elemental phosphorus is the base for furnace-grade phosphoric acid, phosphorus pentasulfide, phosphorus pentoxide, and phosphorus trichloride.[citation...
21 KB (2,488 words) - 20:13, 22 October 2024
appear dark, it is a colorless liquid. It is prepared by treating phosphorus pentasulfide with ethanol: P2S5 + 4 C2H5OH → 2 (C2H5O)2PS2H + H2S Diethyl- and...
4 KB (218 words) - 19:41, 27 October 2022
acetylation. Many related reactions have been demonstrated. For example, phosphorus pentasulfide (P4S10) converts anisole to Lawesson's reagent, [(CH3OC6H4)PS2]2...
9 KB (806 words) - 17:45, 6 November 2024
Organophosphorus chemistry (redirect from Carbon-phosphorus bond)
coordination complex are produced annually by the reaction of phosphorus pentasulfide with alcohols. Phosphoryl thioates are thermodynamically much stabler...
17 KB (1,837 words) - 04:26, 14 November 2024
Thioamides are typically prepared by treating amides with phosphorus sulfides such as phosphorus pentasulfide, a reaction first described in the 1870s. Alternative...
5 KB (500 words) - 15:43, 31 July 2024
reagent Si2Cl6 stereospecifically deoxygenates phosphine oxides. Phosphorus pentasulfide and the related Lawesson's reagent convert certain organic carbonyls...
5 KB (583 words) - 06:47, 15 January 2024
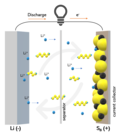
Kang-Jun; Park, Jun-Woo (May 2022). "Multimodal Capturing of Polysulfides by Phosphorus-Doped Carbon Composites for Flexible High-Energy-Density Lithium–Sulfur...
59 KB (5,672 words) - 22:07, 17 October 2024
prepared in a multistep process starting with the base hydrolysis of phosphorus pentasulfide: P2S5 + 6 NaOH → 2 Na3PO2S2 + H2S + 2 H2O The salt is isolated...
4 KB (364 words) - 13:56, 24 January 2024
determination of various ions. It can be obtained by the reaction of phosphorus pentasulfide with ethanol and ammonia. In crystal structure of this compound...
2 KB (112 words) - 13:23, 20 January 2024
is here. Zinc dithiophosphate are produced in two steps. First phosphorus pentasulfide is treated with suitable alcohols (ROH) to give the dithiophosphoric...
8 KB (909 words) - 14:34, 4 January 2024
70 mg/m3 - 10026138 1314-80-3 1407 Phosphorus pentasulfide 250 mg/m3 - 1314803 7719-12-2 0696 Phosphorus trichloride 140 mg/m3 25 ppm 7719122 85-44-9 0315...
93 KB (683 words) - 19:08, 17 June 2024
Thiophosphoryl bromide can be prepared by heating phosphorus tribromide with phosphorus pentasulfide, or with elemental sulfur in an inert atmosphere at...
6 KB (463 words) - 05:50, 11 February 2024
CH3C(O)SH + CH3C(O)OH It has also been produced by the action of phosphorus pentasulfide on glacial acetic acid, followed by distillation. CH3C(O)OH + P2S5...
7 KB (607 words) - 00:35, 22 November 2024
15578–16–2 P4S9 phosphorus nonasulfide 25070–46–6 P4S10 phosphorus pentasulfide 1314–80–3 P4Se3 phosphorus triselenide 1314–86–9 PaBr4 protactinium(IV) bromide...
139 KB (120 words) - 17:07, 15 July 2024
(Ag+, Cu+). Thioacetamide is prepared by treating acetamide with phosphorus pentasulfide as shown in the following idealized reaction: CH3C(O)NH2 + 1/4...
7 KB (489 words) - 12:48, 1 March 2024
using reagents that exchange S and O atoms. A common reagent is phosphorus pentasulfide, although that reagent also tends to induce side-reactions.: 928 ...
8 KB (941 words) - 19:17, 13 September 2024