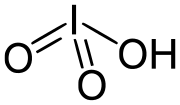
Sodium iodate (NaIO3) is the sodium salt of iodic acid. Sodium iodate is an oxidizing agent. It has several uses. It can be prepared by reacting a sodium-containing...
5 KB (228 words) - 14:09, 24 January 2024
Silver iodate can be obtained by reacting silver nitrate (AgNO3) with sodium iodate or potassium iodate. The by-product of the reaction is sodium nitrate...
3 KB (158 words) - 05:49, 11 September 2024
extracts with sodium bisulfite to give sodium iodide. This comproportionation reaction is a major source of the sodium iodide. Calcium iodate can be produced...
3 KB (187 words) - 11:33, 1 November 2023
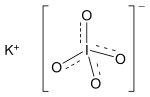
Potassium iodate (KIO3) is an ionic inorganic compound with the formula KIO3. It is a white salt that is soluble in water. It can be prepared by reacting...
11 KB (746 words) - 10:30, 18 April 2024
Sodium chlorate is an inorganic compound with the chemical formula NaClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic...
19 KB (1,809 words) - 06:15, 29 September 2024
especially in commercially prepared solutions, chemically oxidized using sodium iodate. Commonly only enough oxidizer is added to convert one half of the haematoxylin...
31 KB (2,450 words) - 12:15, 19 August 2024
dihydrate can be obtained by reacting bismuth nitrate and potassium iodate or sodium iodate. It is obtained by evaporation and crystallization in 7 mol/L nitric...
3 KB (199 words) - 13:02, 5 September 2024
7 January 2019. Foote, H. W.; Vance, J. E. (1933). "The system; sodium iodate, sodium oxalate, water". American Journal of Science. 26 (151): 16–18. Bibcode:1933AmJS...
7 KB (592 words) - 15:33, 25 October 2024
whose main product is sodium nitrate. In total, they can contain at least 0.02% and at most 1% iodine by mass. Sodium iodate is extracted from the caliche...
104 KB (11,657 words) - 18:48, 27 October 2024
Iodine clock reaction (section Iodate variation)
uses a solution of iodate ion (for instance potassium iodate) to which an acidified solution (again with sulfuric acid) of sodium bisulfite is added....
9 KB (991 words) - 14:03, 26 June 2024
Sodium bromate, the inorganic compound with the chemical formula of NaBrO3, is the sodium salt of bromic acid. It is a strong oxidant. Sodium bromate...
6 KB (283 words) - 12:31, 2 November 2024
Zirconium iodate is an inorganic compound with the chemical formula Zr(IO3)4. It can be prepared by reacting sodium iodate and zirconium sulfate tetrahydrate...
3 KB (190 words) - 17:07, 27 September 2024
bromine and sodium hydroxide: NaIO 3 sodium iodate + Cl 2 + 4 NaOH ⟶ Na 3 H 2 IO 6 + 2 NaCl + H 2 O {\displaystyle {\ce {{\overset {sodium\ iodate...
19 KB (2,111 words) - 17:56, 31 August 2024
industrial scale production involves the oxidation of a solution of sodium iodate under alkaline conditions, either electrochemically on a PbO2 anode...
12 KB (976 words) - 01:21, 2 November 2024
Iodic acid (redirect from Hydrogen iodate)
orange as the indicator. Iodic acid can be used to synthesize sodium or potassium iodate for increasing iodine content of salt.[citation needed] Iodic...
8 KB (644 words) - 02:04, 12 September 2024
Salt (category Sodium minerals)
table salt mixed with a minute amount of potassium iodide, sodium iodide, or sodium iodate. A small amount of dextrose may also be added to stabilize...
71 KB (7,459 words) - 14:37, 4 November 2024
graphite; silicon, from silica; phosphorus, from phosphates; iodine, from sodium iodate; radon, as a decay product from uranium ores; fluorine, from fluorite;...
186 KB (16,953 words) - 11:05, 23 October 2024
increases, and as a result, the concentration of sulfide ions decreases. Barium iodate, Ba(IO3)2, has a solubility product Ksp = [Ba2+][IO3−]2 = 1.57 x 10−9. Its...
7 KB (930 words) - 08:24, 20 October 2023
phosphate NaI sodium iodide 7681-82-5 NaIO sodium hypoiodite NaIO2 sodium iodite NaIO3 sodium iodate NaIO4 sodium periodate NaNH2C6H4SO3 sodium sulfanilate...
183 KB (107 words) - 22:11, 6 September 2024
iodide sources, depending on the producer: potassium iodate, potassium iodide, sodium iodate, and sodium iodide. Any of these compounds supplies the body...
36 KB (4,100 words) - 01:02, 10 November 2024
of sodium hydrogen periodate (Na3H2IO6). This commercially available, but can also be produced by the oxidation of iodates with chlorine and sodium hydroxide...
9 KB (650 words) - 08:21, 24 October 2024
1 9.6 11.1 12.7 16 Sodium hydroxide NaOH 98 109 119 129 174 Sodium iodate NaIO3 2.48 4.59 8.08 10.7 13.3 19.8 26.6 29.5 33 Sodium iodide NaI 159 167 178...
84 KB (196 words) - 10:05, 30 October 2024
– NaOI Sodium hypophosphite – NaPO2H2 Sodium iodate – NaIO3 Sodium iodide – NaI Sodium manganate – Na2MnO4 Sodium molybdate – Na2MoO4 Sodium monofluorophosphate...
119 KB (8,735 words) - 17:31, 25 October 2024
Iodate nitrates are mixed anion compounds that contain both iodate and nitrate anions. Iodate nitrates can have high birefringence. The scandium salt...
8 KB (632 words) - 02:42, 8 November 2023
7681–82–5 NaIO3 sodium iodate 7681–55–2 NaIO4 sodium periodate 7790–28–5 NaNH2 sodium amide 7782–92–5 NaNO2 sodium nitrite 7632–00–0 NaNO3 sodium nitrate 7631–99–4...
139 KB (120 words) - 17:07, 15 July 2024
[I2O8(OH2)]4−, and I 2O4− 9. They are usually made by oxidising alkaline sodium iodate electrochemically (with lead(IV) oxide as the anode) or by chlorine...
30 KB (3,558 words) - 02:08, 12 September 2024
acid. Note that the pronunciation is per-iodate, not period-ate. Unlike other common periodates, such as sodium periodate and periodic acid, it is only...
4 KB (326 words) - 15:56, 29 February 2024
bromate, calcium salts such as calcium iodate, L-cystine, L-cysteine HCl, glycerol monostearate, azodicarbonamide, sodium stearoyl lactylate, sucrose palmitate...
18 KB (2,025 words) - 07:06, 7 November 2024
evaporates. Potassium iodate (KIO3) is used to iodize some salts so that the iodine is not lost by oxidation. Dextrose or sodium thiosulfate are often...
54 KB (5,662 words) - 18:35, 9 November 2024
Sodalite (category Sodium minerals)
been included in sodalite-structure materials include nitrate, iodide, iodate, permanganate, perchlorate, and perrhenate. Minerals portal Labradorite...
17 KB (1,811 words) - 09:40, 15 October 2024