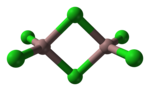
Thorium trichloride is a binary inorganic compound of thorium metal and chlorine with the chemical formula ThCl3. The compound can be prepared by reducing...
4 KB (233 words) - 16:12, 12 May 2024
its chemical compounds. Actinium is found only in traces in uranium and thorium ores as the isotope 227Ac, which decays with a half-life of 21.772 years...
42 KB (4,651 words) - 16:42, 9 August 2024
do, only thorium and uranium do so in more than trace quantities. The most abundant or easily synthesized actinides are uranium and thorium, followed...
130 KB (11,602 words) - 15:30, 15 August 2024
reactor. Another fissile isotope, uranium-233, can be produced from natural thorium and is studied for future industrial use in nuclear technology. Uranium-238...
110 KB (12,436 words) - 13:31, 13 August 2024
Neodymium(III) chloride (redirect from Neodymium trichloride)
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a...
24 KB (2,487 words) - 20:20, 3 January 2024
Thiophosphoryl chloride – Cl3PS Thorium(IV) nitrate – Th(NO3)4 Thorium(IV) sulfate – Th(SO4)2 Thorium dioxide – ThO2 Thorium tetrafluoride – ThF4 Thulium(III)...
119 KB (8,734 words) - 09:23, 30 July 2024
states are the most common. The most prevalent precursor is ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically...
50 KB (5,761 words) - 00:38, 16 August 2024
Nitrogen trifluoride is the only stable nitrogen trihalide, with nitrogen trichloride, nitrogen tribromide, and nitrogen triiodide being explosive—nitrogen...
34 KB (4,010 words) - 23:53, 16 July 2024
of the initial product. AcF3 + 2 NH3 + H2O → AcOF + 2 NH4F Actinium trichloride is obtained by reacting actinium hydroxide or oxalate with carbon tetrachloride...
8 KB (737 words) - 18:34, 12 May 2024
tetrachloride (TiCl4), a component of smoke screens and catalysts; and titanium trichloride (TiCl3), which is used as a catalyst in the production of polypropylene...
79 KB (8,788 words) - 08:10, 13 August 2024
Michelet, Bastien; Bour, Christophe; Gandon, Vincent (2014), "Gallium Trichloride", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons,...
15 KB (1,386 words) - 13:22, 12 July 2024
7446–35–7 ThBr4 thorium(IV) bromide 13453–49–1 ThC thorium(IV) carbide 12012–16–7 Th(CO3)2 thorium(II) carbonate 19024–62–5 ThCl4 thorium(IV) chloride 10026–08–1...
139 KB (120 words) - 17:07, 15 July 2024
Richard (1971). "Crystal structures of americium trichloride hexahydrate and berkelium trichloride hexahydrate". Inorganic Chemistry. 10: 147. doi:10...
76 KB (9,245 words) - 19:49, 16 August 2024
occurring technetium is a spontaneous fission product in uranium ore and thorium ore (the most common source), or the product of neutron capture in molybdenum...
68 KB (7,713 words) - 17:34, 17 August 2024
explosive form of antimony can be formed from the electrolysis of antimony trichloride, but it always contains appreciable chlorine and is not really an antimony...
59 KB (6,840 words) - 02:24, 30 July 2024
polymeric with tetrahedral four-coordinate aluminium centers. Aluminium trichloride (AlCl3) has a layered polymeric structure below its melting point of...
135 KB (14,495 words) - 02:09, 15 August 2024
Structure of W6Cl18 ("tungsten trichloride")...
81 KB (9,128 words) - 15:58, 18 August 2024
Tempelton DH, Carter GF (1954). "The Crystal Structure of Yttrium Trichloride and Similar Compounds". J Phys Chem. 58 (11): 940–943. doi:10.1021/j150521a002...
29 KB (3,545 words) - 08:04, 13 August 2024
produce neptunium trichloride: 6 NpCl4 + 2 NH3 → 6 NpCl3 + 6 HCl + N2 Neptunium tetrachloride can be reduced to neptunium trichloride by hydrogen at 450...
4 KB (299 words) - 12:56, 12 May 2024
is bearing a negative charge, the compound is usually called nitrogen trichloride. Chlorination of metals with Cl2 usually leads to a higher oxidation...
117 KB (13,028 words) - 12:00, 13 August 2024
their acute toxicity; two examples are potassium perrhenate and rhenium trichloride, which were injected as a solution into rats. The perrhenate had an LD50...
44 KB (5,043 words) - 10:22, 13 August 2024
silver perbromate AgCl silver chloride 7783-90-6 AgCl3Cu2 dicopper silver trichloride 69569-03-5 AgClO3 silver chlorate 7783-92-8 AgClO4 silver perchlorate...
182 KB (107 words) - 15:39, 24 July 2024
fluoride 75-02-5 Vinylidene chloride (1,1-Dichloroethylene) 75-35-4 Vinyl trichloride (1,1,2-Trichloroethane) 79-00-5 Vismodegib 879085-55-9 Warfarin 81-81-2...
48 KB (211 words) - 18:08, 25 April 2024
Iodine forms all three possible diatomic interhalogens, a trifluoride and trichloride, as well as a pentafluoride and, exceptionally among the halogens, a...
106 KB (11,790 words) - 10:37, 13 August 2024
oxidation states are stabilized by the larger halogens, so that the trichloride, tribromide, triiodide, and even diiodide are known. The oxidation state...
52 KB (5,851 words) - 09:24, 13 August 2024
Gallium trichloride is a common starting reagent for the formation of organogallium compounds, such as in carbogallation reactions. Gallium trichloride reacts...
74 KB (8,761 words) - 13:06, 13 August 2024
University of California created the first californium compounds—californium trichloride, californium(III) oxychloride, and californium oxide—by treating californium...
44 KB (4,615 words) - 18:40, 29 June 2024
vanadate TlVO3 0.87 Thorium(IV) fluoride ThF4·4H2O 0.914 Thorium(IV) iodate Th(IO3)4 0.03691 Thorium(IV) nitrate Th(NO3)4 186 187 191 Thorium(IV) selenate Th(SeO4)2·9H2O...
84 KB (193 words) - 10:24, 24 July 2024
stable only below −50 °C, at which temperature it decomposes to the trichloride, releasing chlorine gas.) Arsenic is used as the group 5 element in the...
130 KB (14,273 words) - 14:22, 13 August 2024
Green, J. L. (1967). "Crystal structure and lattice parameters of curium trichloride". Journal of Inorganic and Nuclear Chemistry. 29 (11): 2745. doi:10...
66 KB (7,853 words) - 13:15, 13 August 2024