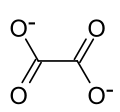
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3. The compound does not dissolve in water...
11 KB (827 words) - 15:31, 5 March 2024
is yttrium(III) oxide (Y 2O 3), also known as yttria, a six-coordinate white solid. Yttrium forms a water-insoluble fluoride, hydroxide, and oxalate, but...
58 KB (6,666 words) - 17:30, 28 September 2024
valence. The solubility properties of yttrium compounds are similar to those of the lanthanides. For example oxalates and carbonates are hardly soluble in...
11 KB (1,244 words) - 20:52, 29 April 2024
Bibcode:1971JCrGr..11..185O. doi:10.1016/0022-0248(71)90083-2. ISSN 0022-0248. "Yttrium oxalate tetrahydrate". Pubchem. National Institutes of Health. Retrieved 5...
5 KB (320 words) - 20:53, 22 August 2024
Rare-earth element (redirect from Yttrium group)
earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths)...
154 KB (16,461 words) - 01:54, 19 September 2024
exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium". Zeitschrift für Chemie. 4 (6): 234–235. ISSN 0044-2402...
3 KB (173 words) - 10:50, 20 December 2023
(1964). "The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium". Zeitschrift für Chemie. 4 (6): 234–235....
2 KB (116 words) - 00:29, 28 June 2024
R.; Matthes, F. The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium. Zeitschrift fuer Chemie, 1964....
3 KB (202 words) - 13:00, 5 February 2024
"Thulium(3+) oxalate". pubchem.ncbi.nlm.nih.gov. Retrieved 29 February 2024. Mamoru Watanabe, Kozo Nagashima (Oct 1971). "Hydrated oxalates of the yttrium group...
3 KB (183 words) - 07:21, 28 March 2024
exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium. Zeitschrift fuer Chemie, 1964. 4 (6): 234-235. ISSN:...
3 KB (200 words) - 10:25, 7 March 2024
Kidney stone disease (section Calcium oxalate)
this oxalate is then excreted in greater amounts into the urine by the kidneys. In the urine, oxalate is a very strong promoter of calcium oxalate precipitation—about...
132 KB (13,802 words) - 19:44, 23 August 2024
the rare-earth elements. It contains the four elements scandium (Sc), yttrium (Y), lutetium (Lu), and lawrencium (Lr). The group is also called the scandium...
53 KB (5,866 words) - 17:54, 11 September 2024
exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium. Zeitschrift für Chemie, 1964. 4 (6): 234-235. ISSN:...
3 KB (160 words) - 20:21, 3 January 2024
Y3Al5O12 Yttrium barium copper oxide – YBa2Cu3O7 Yttrium cadmium – YCd Yttrium copper – YCu Yttrium gold – YAu Yttrium iridium – YIr Yttrium iron garnet...
119 KB (8,735 words) - 14:26, 16 September 2024
An oxalate chloride or oxalato chloride is a mixed anion compound contains both oxalate and chloride anions. Related compounds include oxalate fluorides...
38 KB (2,124 words) - 04:58, 1 June 2024
The carbonate oxalates are mixed anion compounds that contain both carbonate (CO3) and oxalate (C2O4) anions. Most compounds incorporate large trivalent...
24 KB (1,118 words) - 08:05, 13 June 2024
and meteorites. Lutetium usually occurs in association with the element yttrium and is sometimes used in metal alloys and as a catalyst in various chemical...
33 KB (3,950 words) - 15:11, 13 August 2024
Oxalate sulfates are mixed anion compounds containing oxalate and sulfate. They are mostly transparent, and any colour comes from the cations. Related...
40 KB (1,830 words) - 11:04, 27 August 2024
Caesium oxalate (standard IUPAC spelling), or dicesium oxalate, or cesium oxalate (American spelling) is a chemical compound with the chemical formula...
11 KB (790 words) - 00:03, 19 March 2024
coordination chemistry, like yttrium and the other lanthanides. Lanthanum oxalate does not dissolve very much in alkali-metal oxalate solutions, and [La(acac)...
48 KB (6,026 words) - 10:26, 10 September 2024
the solution is treated with ammonium oxalate to convert rare earths into their insoluble oxalates. The oxalates are converted to oxides by annealing....
34 KB (4,095 words) - 04:24, 28 September 2024
and that of neodymium-praseodymium, samarium, gadolinium and yttrium in oxide and oxalate form (with more than 99% Purity). RED also produces strategic...
19 KB (1,829 words) - 14:06, 24 September 2024
spaces below yttrium blank as a third option, but there is confusion on whether this format implies that group 3 contains only scandium and yttrium, or if it...
40 KB (4,504 words) - 07:15, 17 February 2024
a chemical element in 1843. He detected it as an impurity in yttrium oxide, Y2O3. Yttrium and terbium, as well as erbium and ytterbium, are named after...
41 KB (5,005 words) - 19:55, 7 September 2024
The solution is treated with ammonium oxalate to convert rare earth to their insoluble oxalates, the oxalates are converted to oxides by annealing, and...
37 KB (4,830 words) - 15:05, 16 September 2024
remaining solution is treated with ammonium oxalate to convert rare earths into their insoluble oxalates. The oxalates are converted to oxides by heating. The...
45 KB (5,661 words) - 12:30, 8 August 2024
though dysprosium carbonate tetrahydrate (Dy2(CO3)3·4H2O) and dysprosium oxalate decahydrate (Dy2(C2O4)3·10H2O) are both insoluble in water. Two of the...
37 KB (4,332 words) - 12:03, 15 September 2024
calcium citrate, malate, and lactate are highly bioavailable, while the oxalate is less. Other calcium preparations include calcium carbonate, calcium...
47 KB (5,901 words) - 03:24, 8 September 2024
(In total, four elements were named after the village, the others being yttrium, terbium, and erbium.) In 1907, the new earth "lutecia" was separated from...
41 KB (5,200 words) - 10:29, 13 August 2024
(NH4)MgPO4 + 2Na+ Ca2+ Ca2+ forms a white precipitate with ammonium oxalate. Calcium oxalate is insoluble in water, but is soluble in mineral acids. Ca2+ +...
71 KB (7,264 words) - 17:53, 11 September 2024