Caesium chloride or cesium chloride is the inorganic compound with the formula CsCl. This colorless salt is an important source of caesium ions in a variety...
39 KB (3,421 words) - 15:09, 16 December 2024
soluble chlorides is produced, and the insoluble chloride double salts of caesium are precipitated as caesium antimony chloride (Cs 4SbCl 7), caesium iodine...
93 KB (10,025 words) - 17:29, 6 December 2024
is the "interpenetrating primitive cubic" structure, also called a "caesium chloride" or B2 structure. This structure is often confused for a body-centered...
49 KB (3,620 words) - 14:56, 4 October 2024
this forms highly toxic/corrosive hydrofluoric acid. The caesium ion (Cs+) and caesium chloride are generally not considered toxic. Haynes, William M.,...
10 KB (898 words) - 23:26, 19 December 2024
be a white solid and is soluble in water. Its properties resemble caesium chloride. It is produced by the reaction of hydrochloric acid with francium...
3 KB (93 words) - 02:30, 17 August 2024
hydrochloric acid to precipitate insoluble thallium(I) chloride. This solid crystallizes in the caesium chloride motif. The low solubility of TlCl is exploited...
6 KB (376 words) - 01:52, 1 May 2024
alternatives. Also the higher specific activity caesium sources tend to be made from very soluble caesium chloride (CsCl), as a result if a radiography source...
36 KB (3,877 words) - 16:30, 25 November 2024
yielded 7.3 grams of caesium chloride and 9.2 grams of rubidium chloride. Rubidium was the second element, shortly after caesium, to be discovered by...
40 KB (4,527 words) - 13:45, 26 December 2024
chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1...
214 KB (23,524 words) - 08:55, 14 December 2024
Caesium iodide or cesium iodide (chemical formula CsI) is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray...
9 KB (653 words) - 13:50, 24 September 2024
around 8 °C, the crystal structure is of the caesium chloride type, with the ozonide ion in place of the chloride ion. At lower temperatures, the crystal structure...
4 KB (314 words) - 11:34, 8 December 2023
Sodium chloride /ˌsoʊdiəm ˈklɔːraɪd/, commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium...
36 KB (3,751 words) - 06:51, 13 December 2024
Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous...
31 KB (2,923 words) - 12:37, 27 December 2024
Caesium cadmium chloride (CsCdCl3) is a synthetic crystalline material. It belongs to the AMX3 group (where A=alkali metal, M=bivalent metal, X=halogen...
3 KB (178 words) - 04:25, 24 November 2024
Goiânia accident (category Caesium)
about 93 grams (3.3 oz) of highly radioactive caesium chloride (a caesium salt made with a radioisotope, caesium-137) encased in a shielding canister made...
42 KB (4,475 words) - 18:29, 3 January 2025
structures that depend on the cation (A+) added. Caesium chloride treated with titanium(II) chloride and hexachlorobenzene produces crystalline CsTi2Cl7...
12 KB (1,204 words) - 05:08, 9 December 2024
RbCl adopts the caesium chloride (CsCl) structure (NaCl and KCl undergo the same structural change at high pressures). Here, the chloride ions form a simple...
11 KB (845 words) - 04:32, 28 May 2024
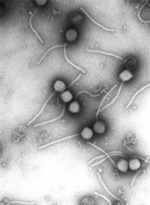
discrete layers of, often visible, concentrated viruses in the tube. Caesium chloride is often used for these solutions as it is relatively inert but easily...
58 KB (7,208 words) - 21:01, 17 November 2024
octahedron. In caesium chloride each caesium has 8 chloride ions (at 356 pm) situated at the corners of a cube and each chloride has eight caesium ions (also...
19 KB (2,209 words) - 02:06, 21 December 2024
Caesium auride is the inorganic compound with the formula CsAu. It is the Cs+ salt of the unusual Au− anion. CsAu is obtained by heating a stoichiometric...
4 KB (301 words) - 05:26, 24 November 2024
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound (with certain covalent characteristics), although the...
14 KB (1,012 words) - 04:57, 17 January 2024
7.35 Halite CsF 6.02 Halite CsCl 4.123 Caesium chloride CsBr 4.291 Caesium chloride CsI 4.567 Caesium chloride Al 4.046 FCC Fe 2.856 BCC Ni 3.499 FCC...
16 KB (1,177 words) - 16:39, 20 March 2024
aurides, compounds containing the Au− anion. Caesium auride (CsAu), for example, crystallizes in the caesium chloride motif; rubidium, potassium, and tetramethylammonium...
141 KB (15,938 words) - 06:23, 6 December 2024
accident in an abandoned Cs-137 source where the caesium was present in easily water-soluble caesium chloride form). However, a sufficiently chemically skilled...
66 KB (7,205 words) - 15:43, 28 December 2024
Caesium bromide or cesium bromide is an ionic compound of caesium and bromine with the chemical formula CsBr. It is a white or transparent solid with...
5 KB (300 words) - 20:49, 12 May 2024
example, halides with the caesium chloride structure (coordination number 8) are less compressible than those with the sodium chloride structure (coordination...
63 KB (6,938 words) - 06:54, 28 December 2024
and Cl− anions, so, the compound is not caesium pentachlorozincate, but caesium tetrachlorozincate chloride. No compounds containing the [ZnCl6]4− ion...
40 KB (4,083 words) - 22:12, 1 January 2025
coordination geometry, in which each ion has a coordination number of six. Caesium chloride, bromide, and iodide crystallize in a body-centered cubic lattice that...
5 KB (314 words) - 16:33, 25 October 2023
studies. Goiânia accident (Brazil), 13 September 1987. An unsecured caesium chloride radiation source left in an abandoned hospital was recovered by scavenger...
33 KB (2,030 words) - 09:29, 8 December 2024
Antineoplaston therapy Apitherapy Black salve Cancell Cell therapy Caesium chloride Chelation therapy Coley's toxins Colloidal silver Coral calcium...
42 KB (4,102 words) - 17:25, 18 December 2024