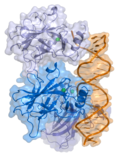
Gendicine is a gene therapy medication used to treat patients with head and neck squamous cell carcinoma linked to mutations in the TP53 gene. It consists...
7 KB (447 words) - 06:36, 11 October 2024
trials were conducted, with more than half of them in phase I. In 2003, Gendicine became the first gene therapy to receive regulatory approval. Since that...
176 KB (18,235 words) - 06:24, 10 November 2024
a viable cancer treatment option. The first commercial gene therapy, Gendicine, was approved in China in 2003 for the treatment of head and neck squamous...
124 KB (13,220 words) - 11:01, 30 August 2024
imatinib 2002 – The State Food and Drug Administration of China approves Gendicine, gene therapy for cancer 2002 – Corporate takeover of Dupont by BMS resulted...
27 KB (2,580 words) - 18:15, 7 September 2023
settings. In 2003, China approved the first gene therapy for clinical use: Gendicine, an adenoviral vector encoding p53. In 2012, the European Union issued...
48 KB (5,532 words) - 12:43, 9 October 2024
first gene therapy product to be licensed to treat cancer, Gendicine, is an adenovirus. Gendicine, an adenoviral p53-based gene therapy was approved by the...
36 KB (4,918 words) - 09:36, 27 February 2024
However, cetuximab's efficacy is still under investigation by researchers. Gendicine is a gene therapy that employs an adenovirus to deliver the tumor suppressor...
105 KB (11,717 words) - 08:56, 13 November 2024
a step forward and a step back; first gene therapy drug was approved, Gendicine, which was approved in China for the treatment of certain cancers, but...
32 KB (3,911 words) - 03:05, 15 September 2024
Exagamglogene autotemcel (Casgevy): treatment for sickle cell disease. Gendicine: treatment for head and neck squamous cell carcinoma Idecabtagene vicleucel...
11 KB (834 words) - 06:28, 10 November 2024